Canada Gazette, Part I, Volume 151, Number 48: Regulations Amending the Patented Medicines Regulations
December 2, 2017
Statutory authority
Patent Act
Sponsoring department
Department of Health
REGULATORY IMPACT ANALYSIS STATEMENT
(This statement is not part of the Regulations.)
Executive summary
Issues: The Patented Medicine Prices Review Board (“PMPRB” or “the Board”) uses a regulatory framework that currently falls short of its mandate to protect Canadian consumers from excessive prices for patented medicines. Canada's patented medicine prices are among the highest in the world, and despite significant changes in the medicine market, the Patented Medicines Regulations have not been substantively changed in over two decades. The Regulations need to be modernized to provide the PMPRB with more relevant and effective regulatory tools in order to better protect Canadians from excessive prices for patented medicines.
Description: This proposal would amend the Patented Medicines Regulations (“Regulations”) so that the PMPRB's regulatory framework includes new price regulatory factors and patentee price information reporting requirements that will help the PMPRB to protect Canadian consumers from excessive prices. There are five elements.
New price regulatory factors and updating the schedule of comparator countries
(1) Providing the PMPRB with three new price regulatory factors to enable it to consider the price of a patented medicine in relation to its value to patients and impact on the health care system.
(2) Updating the schedule to the Regulations that sets out the countries (now the PMPRB7) on which patentees report pricing information to include countries with similar consumer protection priorities, economic wealth, and marketed medicines as Canada. This would provide the PMPRB with the information needed to regulate prices based on comparisons that are more closely aligned with the PMPRB's mandate and Canada's domestic policy priorities.
New reporting requirements
(3) Reducing reporting obligations for patented veterinary, over-the-counter and “generic” medicines (i.e. those authorized for sale by the Minister of Health through an Abbreviated New Drug Submission [ANDS]). As these products pose a lower risk of asserting market power and charging excessive prices, this reduction would enable the PMPRB to focus on medicines at higher risk of excessive pricing.
(4) Amending patentee price information reporting requirements to include reporting in relation to the new factors.
(5) Requiring patentees to report price and revenue information net of all price adjustments such as direct or indirect third party discounts or rebates. This would ensure that the PMPRB is fully informed of the actual prices for patented medicines in Canada and enhance the relevance and impact of domestic price comparisons.
Cost-benefit statement: The proposed amendments would produce an estimated net benefit to Canadians of $12.6 billion net present value (NPV) over 10 years due to reduced prices for patented medicines. Lower prices would alleviate financial pressures on public and private insurers and improve affordable access for Canadians paying out-of-pocket. Lost revenues to industry are estimated to be $8.6 billion present value over 10 years. Costs to industry are estimated to be $9K/year in total, including administrative and compliance costs. Government costs of approximately $8.8M/year (PV) would include increasing the PMPRB's staff and resources for an anticipated increase in compliance and enforcement activities.
It is not anticipated that these amendments would generate adverse impacts on industry employment or investment in the Canadian economy. Although when the current regulatory framework was first conceived 30 years ago, policy makers believed that patent protection and price were key drivers of medicine research and development (R&D) investment, there is no evidence of this link. The level of industry R&D investment relative to sales by medicine patentees in Canada has been falling since the late 1990s and is now at a historic low despite Canada having among the highest patented medicine prices in the world. These amendments would aim to align Canadian prices with those in countries that, despite having lower prices, receive higher medicine industry investment.
“One-for-One” Rule and small business lens: The “One-for-One” Rule applies and the anticipated administrative burden is estimated to be $3,062 (2012 dollars) annually. The small business lens does not apply.
Domestic and international coordination and cooperation: Price regulations on medicines are a common international practice, although there is a significant variation in approach. These differences often arise from a need to tailor policy instruments to work within each country's health care system. While countries monitor foreign models, it is to keep abreast of international best practices, rather than to harmonize. Regulating the prices for patented medicines to be non-excessive is not subject to trade provisions.
Background
Patented medicines are an important part of Canada's health care system
Patented medicines help prevent and cure disease as well as save lives. But Canadians are not getting the value for money on prescription medicine spending or the outcomes they deserve. Medicine spending in Canada has increased from less than 10% of total health expenditure, when Medicare was first established 49 years ago, to about 16% today. Medicines are now the second-largest category of spending in health care, ahead of physician services and behind total hospital spending (which includes medicines used in hospital). Canadians are spending more per capita on medicines than any other country in the world, with the exception of the United States. Greater medicine expenditures can limit access to innovative medicines by straining the budget envelope for medicines of public and private insurers, place a financial burden on patients who pay out of pocket for their medicines, and mean fewer resources for other critical areas of the health care system.
In January 2016, federal, provincial and territorial ministers agreed to work together to improve the accessibility, affordability, and appropriate use of medicines to better meet health care system needs. The Government of Canada is committed to this work and is taking action to lower the cost of medicines, provide faster access to new medicines that are safe and effective, and support the development of tools for more appropriate prescribing. To support these actions, Budget 2017 outlined an investment of $140.3 million over five years, starting in 2017–2018, and $18.2 million, for ongoing years. The proposed regulatory amendments contribute to this initiative with respect to the price of patented medicines.
The Patented Medicine Prices Review Board (“PMPRB” or “the Board”)
The PMPRB was created in 1987 as the consumer protection “pillar” of a major set of reforms to the Patent Act (“Act”), which were designed to encourage greater investment in medicine R&D in Canada through stronger patent protection. The Act sets out the period of time that patentees of a medicine are provided the exclusive rights granted by a patent. It also establishes the PMPRB as a quasi-judicial body with a price regulatory mandate to ensure that patentees do not abuse their patent rights by charging consumers excessive prices during this statutory monopoly period.
The Act and the Patented Medicines Regulations (“Regulations”) together form the patented medicines price regulatory framework of the PMPRB. Regulations with respect to patented medicine prices and information are made pursuant to the Minister's recommendation; however, the PMPRB carries out its regulatory mandate at arm's length from the Minister.
The Patent Act and Patented Medicines Regulations
Although no definition of “excessive” is included in the regulatory framework, it does specify the factors and information that the Board must consider in determining whether a price is excessive. The current price regulatory factors as set out in section 85 of the Act are the following:
- The prices at which the same medicine has been sold in the relevant market;
- The prices at which other medicines in the same therapeutic class have been sold in the relevant market;
- The prices at which the medicine and other medicines in the same therapeutic class have been sold in countries other than Canada; and
- Changes in the Consumer Price Index.
The Regulations specify the price information that patentees must report to the PMPRB to allow it to regulate prices and report on trends. They include requirements to report the identity and price information for patented medicines sold in Canada and their prices in seven foreign countries where they are also sold. Currently the seven countries set out in the schedule to the Regulations (the PMPRB7) are the United States, the United Kingdom, France, Germany, Switzerland, Italy and Sweden. Although section 85 of the Act allows for further price regulatory factors to be prescribed in the Regulations, none have been proposed for consideration until now.
The PMPRB's Compendium of Policies, Guidelines and Procedures
Many of the core regulatory concepts in the Act and the Regulations have been further developed in, and are operationalized through, guidelines. The PMPRB is authorized to make non-binding guidelines under section 96 of the Act, subject to consultation with relevant stakeholders. The purpose of the guidelines is to establish, and ensure that patentees are generally aware of, the policies and procedures undertaken by the Board staff to identify the medicines that might be priced excessively.
How the current regulatory framework works
Under the PMPRB's current regulatory framework, as operationalized through the guidelines, new patented medicines are assessed for the degree of therapeutic benefit they provide relative to existing medicines on the market. Depending on the outcome of that process, the PMPRB determines a price ceiling for new patented medicines that is based either on the median price of that same medicine in the PMPRB7 countries, the highest-priced medicine in Canada in the same therapeutic class, or some combination of the two. Once a patentee sets a medicine's introductory price in relation to that ceiling and it enters the market, the PMPRB allows annual price increases in keeping with the Consumer Price Index (CPI), provided these increases do not make the Canadian price greater than the highest price of the same medicine among the PMPRB7 countries.
The PMPRB's current regulatory framework is operationalized by Board staff who investigate medicines that appear to be priced excessively. Board staff apply the tests and thresholds specified in the guidelines to each patented medicine sold in Canada, notify the patentee that they are under investigation if the prices fail those tests and thresholds, and try to negotiate a voluntary compliance undertaking (VCU) by the patentee based on the compliant price level as set out in the guidelines. A VCU is a written commitment by a patentee to comply with the PMPRB's guidelines, including adjusting the price of the patented medicine in question to a level that complies with the guidelines and offsetting any potential excess revenues that may have been received as the result of having sold the patented medicine at a non-guideline compliant price in Canada.
If an acceptable VCU is not concluded, the case proceeds to a public adversarial hearing in front of a panel composed of members of the Board. During a hearing, the Board panel acts as a neutral arbiter between the parties (Board staff and the patentee). The Board panel must consider every factor under subsection 85(1) in determining whether the price of a medicine sold in Canada is excessive. The Board panel is not bound by the guidelines during a hearing, although the Board staff, when presenting evidence in front of the Board, often relies on tests and methods that appear in the guidelines as part of its case that the medicine has been sold at an excessive price. If the Board panel determines that the medicine was sold at an excessive price, it may issue an order to enforce a non-excessive price and order the patentee to repay any excess revenue that resulted from selling the drug at an excessive price. An order of the Board can be enforced in the same manner as an order of the Federal Court.
Canada's changing market and rising medicine costs
Since the establishment of the PMPRB three decades ago, the medicine market has changed significantly. Medicine development is increasingly focussed on higher-cost medicines, such as biologics, genetic therapies targeted to smaller patient populations and medicines for rare diseases. The risk of asserting market power through excessive pricing is often greater for these products since there are few, if any, substitutes, and the patentee is not subject to competition. This is especially true for medicines that are first of their kind, or for which alternatives are less effective or have less tolerable side effects.
The current market dynamic has contributed to a significant increase in the cost of medicine in Canada which, if left unaddressed, is expected to continue. Between 2005 and 2016, the number of medicines in Canada with annual per-patient treatment costs of at least $10,000 increased from 20 to 135. This represents between 30% and 40% of new patented medicines coming under the PMPRB's jurisdiction each year and is a dramatic increase in these types of medicines over a brief timeframe. In 2015, 20 medicines had annual per-patient treatment costs over $50,000. High-cost specialty medicines now account for nearly one quarter of public and private insurer costs, but less than 1% of their beneficiaries.
Canadian patented medicine prices are among the highest in the world. Of all 35 Organisation for Economic Co-operation and Development (OECD) member countries, only the United States and Mexico have higher patented medicine prices than Canada. In 2015, median OECD prices for patented medicines were on average 22% below those in Canada.
Confidential price adjustments
Medicine manufacturers increasingly negotiate price adjustments with insurers in exchange for having their products reimbursed through insurance plans. These price adjustments are typically negotiated in confidence, with the agreement that they not be disclosed publicly. This means that there is a growing discrepancy between public list prices and lower actual prices paid in the market due to the increased use of confidential price adjustments.
Limitations of current price regulation
For the past 20 years, many countries that set price limits on medicines have relied on international price comparison between countries. With the emergence of higher-cost medicines, coupled with confidential price adjustments, countries have had to modernize with new methods that, for those medicines, are more reliant on assessing the economic value of a new medicine to their respective health systems and less on comparing prices internationally. Between 2010 and 2012, 23 European countries began planning or executed significant reforms to their regulatory frameworks for patented medicine prices. While international price comparison is still widely used in international price regulation, it is increasingly used as an adjunct to other pricing factors.
Price regulatory factors
Section 85 of the Act sets out the price regulatory factors that the Board must consider in determining whether a medicine is being or has been sold at an excessive price in Canada. The current price regulatory factors direct the Board to consider the prices at which a medicine or other medicines in the same therapeutic class have been sold in other countries. The PMPRB relies upon public prices when making price comparisons internationally; however, these public prices do not reflect the confidential price adjustments negotiated with some insurers that have become systemic in Canada and around the world. In an era marked by high-cost specialty medicines, the level of confidential price adjustments negotiated can be substantial. This means that there is a growing discrepancy between public list prices and lower actual prices paid in the market and leaves the PMPRB to regulate on the basis of public prices that bear less and less resemblance to what insurers are actually paying in the market. The PMPRB needs other factors that it can use to assess whether a price is excessive.
The schedule of comparator countries
The schedule to the Regulations sets out the seven countries for which patentees are to submit price information. The PMPRB uses the prices of the same patented medicines in these countries, where available, to set price limits on medicine prices in Canada at introduction and in subsequent years. The schedule of countries to the Regulations has not been updated since the Regulations were first conceived 30 years ago. At that time, policy makers believed that patent protection and price were key drivers of medicine R&D investment. The choice was made to offer a comparable level of patent protection and pricing for medicines as existed in countries with a strong medicine industry presence, on the assumption that Canada would come to enjoy comparable levels of R&D. However, the percentage of R&D-to-sales by patentees in Canada has been falling since the late 1990s and is currently less than Canada obtained at the time of the 1987 Patent Act reforms. By comparison, and despite Canada having among the highest patented medicine prices, industry R&D investment relative to sales in the PMPRB7 countries is on average 22.8% versus 4.4% in Canada. As a result, there is no evidence of a determinant link between domestic prices and the location of industry R&D investment. Other factors, such as head office location, clinical trials infrastructure and scientific clusters, appear to be much more influential determinants of where medicine investment takes place in a global economy.
The policy intent of the original schedule selection has not materialized and is no longer considered to be the most appropriate basis for the composition of the countries listed in the schedule. The regulatory requirements for patentees to report on prices in the PMPRB7 keep Canadian prices for patented medicines among the highest in the world.
Issues
The Board determines whether a price is excessive based on the price regulatory factors in the Act, and the patentee price information reporting requirements specified in the Regulations. The evolution in the global and Canadian medicine environment has made apparent two important limitations to the Board's current regulatory framework: (1) the ineffectiveness of the current price regulatory factors to adequately inform the PMPRB's assessment of excessiveness; and (2) the insufficiency of the patentee price information reporting requirements.
Under the current regulatory framework, excessiveness is assessed almost entirely on the basis of domestic and international public list prices. This is problematic with an influx in high-cost specialty medicines and list prices not reflective of what public and private insurers are actually paying. The main limitations of the current framework are that
- It does not provide additional price regulatory factors, beyond price comparisons and CPI, for the PMPRB to assess whether a price is excessive. It does not consider whether the price of a medicine reflects
- The value of a medicine to a patient: medicines that offer substantial clinical benefits to patients or are alone in their therapeutic class will be in greater demand than medicines that are only marginally better than the standard of care or are one among many in their class;
- The number of patients that can benefit from a medicine: the size of the market for a medicine can have an impact on its expected price and the ability to pay for the medicine in a given country; and
- The wealth of a country: countries with greater economic resources can afford more or higher-cost medicines than countries with fewer resources.
- The list of countries used for price comparisons (PMPRB7) is out of date. Canadian prices for new medicines are compared to those of countries with high medicine prices, rather than to those of countries with similar medicine markets, consumer protection and wealth. The selection of countries can have a significant impact on the price maximums for patented medicines in Canada. As the PMPRB relies on international price comparisons, the PMPRB7 set of comparator countries has the effect of allowing higher prices in Canada than would otherwise be the case if comparator countries were more reflective of the Canadian medicine market.
Objectives
The proposed amendments to the Patented Medicines Regulations would ensure that the PMPRB is equipped with the price regulatory factors and patentee price information reporting requirements necessary to fulfill its mandate to protect Canadian consumers from excessive prices for patented medicines. It is anticipated that the implementation of these amendments by the PMPRB would lead to lower prices for patented medicines in Canada that are more closely aligned with their value to patients and the health care system, and Canadians' willingness and ability to pay.
Description
There are five elements included in the proposed amendments.
Price regulatory factors and updating the schedule of comparator countries
- Introduce new, economics-based price regulatory factors that would enable the PMPRB to ensure non-excessive prices that reflect value and Canada's willingness and ability to pay for patented medicines.
- Update the schedule of countries used by the PMPRB for international price comparisons to be better aligned with the consumer protection mandate of the PMPRB and median OECD prices.
Reporting requirements
- Reduce reporting obligations for patented veterinary, over-the-counter and “generic” medicines.
- Set out the information reporting requirements to enable the PMPRB to operationalize the new price regulatory factors.
- Require patentees to report price and revenue information that is net of all domestic price adjustments such as direct or indirect third party discounts or rebates and any free goods or services.
A more detailed description of each of the proposed amendments follows.
1. Introduce new, economics-based price regulatory factors that would ensure prices reflect value and Canada's willingness and ability to pay for patented medicines
This proposed amendment would introduce three additional price regulatory factors of pharmacoeconomic value, market size, and gross domestic product (GDP) and GDP per capita in Canada. These new price regulatory factors would enable the PMPRB to consider complementary and highly relevant aspects of price excessiveness related to the value of the health benefit produced by the medicine, and the willingness and ability of Canadian consumers to pay for it. These new factors will only apply to sales of patented medicines that occur after the coming into force of the proposed amendments.
Pharmacoeconomic value of the medicine in Canada
The price paid for a medicine should take into consideration the value it produces. At the same time, it must recognize the cost to supply the medicine if manufacturers of medicines are to continue to invest in the production of new medicines. A pharmacoeconomic evaluation identifies, measures, and compares the costs and benefits of a given medicine to patients and the health care system. The inclusion of this factor would require the Board to consider whether a medicine's price is commensurate with the benefits it provides to patients within the context of the Canadian health care system.
Size of the market for the sale of the medicine in Canada and in countries other than Canada
The addition of this factor in the Regulations could enable the PMPRB to develop market impact tests for medicines that are likely to pose affordability challenges for insurers due to the market size for the medicine. The impact of an excessive price is a function of both price and volume; the larger the size of the market for the medicine in Canada, the greater the impact of its price. Where public and private insurers are called on to cover the cost of a medicine for a significant number of patients, the high cost of a medicine could render the medicine unaffordable for all who need it. The Canadian price could be assessed against international prices and prevalence (number of people with the disease) levels in an effort to evaluate the price-volume relationship and establish a reasonable market impact test. Including the size of the market as a factor would also allow the PMPRB to reassess the prices of patented medicines over time. Once a medicine is on the market, the patentee may seek regulatory approval from Health Canada to use the medicine in the treatment of other conditions, or the medicine might also be prescribed by physicians off-label (i.e. prescribed for the treatment of conditions for which the medicine has not received regulatory approval). Since patented medicines are protected from new entrants, their prices can remain unaffected from subsequent fluctuations in the size of the market into which they may be sold. As patentees are assumed to set their introductory prices at a profitable level to recoup initial investment, a growth in the market size should align and correct prices downwards to a comparable level. Failure to do so could suggest that the original price, for an expanded market, is now excessive.
GDP in Canada and GDP per capita in Canada
The GDP is a measure of a country's economic output. GDP growth measures how much the inflation-adjusted market value of the goods and services produced by an economy is increasing over time. Per capita GDP measures how much a country is producing relative to its population. Growth in Canadian GDP can be taken as an indicator of the country's ability to pay year-over-year, whereas per capita GDP is a proxy for buying power at the level of the individual. The introduction of GDP in Canada and GDP per capita in Canada as a price regulatory factor would provide the PMPRB with measures of ability to pay for medicines at the national and individual level. The inclusion of this factor would allow the PMPRB to assess the impact of a medicine's price on the finances of consumers and insurers. It could also enable the PMPRB to develop market impact tests for medicines that are likely to pose affordability challenges for insurers due to the market size for the medicine.
2. Update the schedule of countries used by the PMPRB for international price comparisons to be better aligned with the PMPRB's consumer protection mandate and median OECD prices
The PMPRB uses the publicly available list prices of patented medicines sold in the PMPRB7 to set maximum prices for the same patented medicines in Canada at introduction and in subsequent years. Depending on their price levels, the selection of countries can have a significant impact on the maximum prices for patented medicines in Canada.
This proposed amendment would reconsider the PMPRB7 to update the list of countries set out in the schedule to be better aligned with the PMPRB's consumer protection mandate, and Canada's wealth and status as a major market for medicines. The scope of countries considered for the revised schedule was the 35 OECD countries, as they share the same economic and social policies as Canada. Requiring patentees to report on prices in all 35 member countries was deemed unnecessary because (1) this would present a significant reporting burden; (2) some OECD countries are better aligned with Canada's domestic policy priorities and economic standing; and (3) it may be difficult to obtain price and sales information from some countries. Three criteria were used to select a subset of OECD countries to form the revised schedule.
First, the countries must have medicine pricing policies that are well aligned with the consumer protection mandate of the PMPRB, such as a country having national pricing containment measures to protect consumers from high medicine prices. For example, the United States does not satisfy this criterion.
Second, countries must possess reasonably comparable economic wealth as Canada, such as a country having a similar economic standing to Canada, as measured by GDP per capita. This is to ensure that prices correspond to Canada's ability to pay for medicines. For example, Canada's GDP per capita ranks eleventh among OECD countries, but prices for patented medicines are the third highest. The proposed schedule includes countries that have reasonably higher, similar and lower GDP per capita as Canada.
Third, countries are required to have a similar medicine market size characteristics as Canada, such as population, consumption, revenues and market entry of new products. This is to ensure that the resulting similar-sized markets produce a price level that is commensurate with Canada's share of global medicine sales.
Using these criteria, the proposed schedule lists Australia, Belgium, France, Germany, Italy, Japan, the Netherlands, Norway, South Korea, Spain, Sweden and the United Kingdom (PMPRB12). Including a larger number of countries in the schedule would make price tests less sensitive to the influence of countries with prices that are high or low, and reduce the impact where price and sales information is delayed or not available. For example, with only seven reference countries, delayed or missing price information from just two of the reference countries could impact the sample median by as much as 10%. Increasing the schedule to 12 countries would reduce this impact to just 2%. This slightly larger list would provide the PMPRB with a more balanced perspective of prevailing market prices and greater stability of the sample median without imposing significantly greater reporting requirements on patentees or administrative burden on the PMPRB.
3. Reduce reporting obligations for patented veterinary, over-the-counter and “generic” medicines
The Regulations currently only require patented veterinary and over-the-counter medicines (that do not contain a controlled substance or are not a radiopharmaceutical or biologic as per the Food and Drugs Act and the Food and Drug Regulations) to report price and sales information to the PMPRB on a complaints basis. Proposed amendments would further reduce reporting obligations for these medicines so that price, sales, and identity information would only be required on request by the PMPRB for all patented veterinary and over-the-counter medicines, including those that may contain a controlled substance, or are a radiopharmaceutical and/or a biologic. Amendments would also extend the same reduced reporting obligations to patented generic medicines (i.e. medicines approved by means of an ANDS). Patentees of generic medicines typically face greater competition, and the risk of excessive pricing due to market power is generally not cause for concern. These proposed amendments are intended to spare patentees unnecessary reporting regulatory burden for medicines that pose a lower risk of excessive pricing. It would also allow the PMPRB to focus its resources on medicines that pose a more substantive risk of excessive pricing.
4. Set out the patentee pricing information reporting requirements to enable the PMPRB to operationalize the new pricing factors
The current Regulations specify what information patentees must provide to the PMPRB in support of the current price regulatory factors. This includes information about the prices of patented medicines sold in Canada and other countries, patentees' revenues and R&D expenditures. Patentees would be required to report new information to the PMPRB to support the new pharmacoeconomic value and market size factors. Patentees would not be required to report on information related to GDP and GDP per capita, as this information would be obtained from Statistics Canada.
Information regarding pharmacoeconomic value: patentees would be required to provide the PMPRB with all published cost-utility analyses that express the value in terms of the cost per quality-adjusted life year (QALY). Cost-utility analyses are viewed by experts as the “gold standard” approach to considering the economic value of new medicines. The cost per QALY quantifies benefit by measuring lengthened life and/or improved quality of life. It is the most established measure of pharmacoeconomic value, as it enables comparisons across different types of medicines by using a common unit of measurement. This information reporting requirement would enable the PMPRB to consider the introduction of the concept of a maximum cost per QALY threshold in Canada.
In recognition of the significant expertise that can be necessary to prepare and validate cost-utility analyses, reporting would be limited to those that have been prepared by a publicly funded Canadian organization, such as the Canadian Agency for Drugs and Technologies in Health (CADTH) or the Institut national d'excellence en santé et services sociaux (INESSS). These organizations have dedicated expertise, and they generally conduct pharmacoeconomic analyses for medicines seeking to be reimbursed by public insurers. The PMPRB would consider these analyses in its evaluation of price excessiveness. It would not duplicate the work conducted by CADTH and INESSS as part of reimbursement processes.
Even though the new pharmacoeconomic value factor would only apply to sales of patented medicines made after the coming into force of the amended Regulations, the obligation to submit the most recently published cost-utility analysis would extend to all patented medicines, both those marketed as of the date of the amended Regulations coming into force and any new medicines offered for sale following the date of the coming into force. Cost-utility analyses are typically only prepared for a given medicine following certain trigger points in a medicine's life cycle (e.g. at time of initial market launch or following regulatory approval for use of the medicine in the treatment of a new condition). Although the most recent cost-utility analysis for an existing medicine could be several years old, it would still reflect the most recent and relevant information for the PMPRB to consider when applying the new factor of pharmacoeconomic value. Patentees would only be required to provide published analyses — there would be no obligation on the patentee to prepare a cost-utility analysis if one does not exist.
Information respecting market size: patentees would be required to provide the PMPRB with information on the estimated maximum use of the medicine in Canada, by quantity of the medicine sold in final dosage form, for each dosage form and strength that are expected to be sold. It is expected that patentees already construct this estimate as part of their development plans to introduce a new patented medicine to the Canadian market. Patentees compile this information in the development of business plans and for CADTH processes. Before going to market, patentees rely upon available statistics and information on the prevalence (number of people with a disease) in a given country and incidence (estimated number of new cases each year) to develop a sales forecast. They also take into account other factors such as competition to estimate the potential market share for their new medicine.
Patentees would also be required to provide the PMPRB with updated estimates that may occur, for example, when a medicine receives approval from Health Canada for use in the treatment of a new condition that expands the estimated market for the medicine. The new factor of market size would only apply to sales of patented medicines made after the coming into force of the amended Regulations. However, in view of the fact that it can take up to three years for the market for a new medicine to fully mature, patentees of medicines that are already on the market and were first offered for sale within three years prior to the amended Regulations coming into force or have received regulatory approval for use in the treatment of a new condition within this same three-year period would be required to provide information on the estimated maximum use of these medicines in Canada.
5. Require patentees to report price and revenues, net of all price adjustments
The Regulations currently require patentees to report information on price adjustments for the first point of sale only. Patentees are not required to report the significant price adjustments they may provide to third party insurers such as provincial insurers that provide reimbursement for the cost of a medicine sold to a patient. Provincial insurers are some of the biggest payers of patented medicines in Canada. Without this information, the PMPRB sets the non-excessive price maximum of a medicine on the basis of information that only includes some price adjustments. This amendment would require patentees to report price and revenue information that is net of any price or other adjustments, including discounts, rebates and free goods and services, to any party that pays for, or reimburses, the medicine. Although most adjustments are likely to result in a price reduction, this amendment is intended to capture information on any adjustment including those resulting in a price increase. This information would be considered privileged as per section 87 of the Patent Act and would be considered by the Board when determining excessiveness.
With this information, the PMPRB would use the price that is net of any price adjustments to calculate the non-excessive price maximum. The PMPRB currently regulates the non-excessive price of a medicine based on the prices of other medicines in the same therapeutic class for sale in Canada. Since that price information does not include third-party price adjustments, the prices of comparator products that subsequently enter the market are often inflated (as the price ceilings for those medicines are determined in relation to an inflated list price of the existing medicine, rather than the actual price paid in Canada). As a result, the therapeutic class comparison tests yield price maximums that are higher than they would be if the actual price paid were available to the PMPRB. Compelling actual price information, inclusive of all price adjustments provided by the patentee, would allow the PMPRB to include rebates in the calculation of the average transaction price. It would also provide a mechanism for patentees to comply with the regime by calculating a true transaction price reflective of all rebates and discounts, direct and indirect.
Regulatory and non-regulatory options considered
Status quo
The option of taking no action was considered and rejected on the grounds that the PMPRB's current regulatory framework lacks effective price regulatory factors and sufficient patentee price information reporting requirements. The current factors do not take into account all the aspects of excessiveness for new categories of medicines that have emerged since the creation of the PMPRB. The PMPRB's current patentee price information reporting requirements produce incomplete domestic pricing information and provide international price information from a number of countries with high patented medicine prices that are not equivalent to the Canadian market.
Non-regulatory modernization (updates to the PMPRB's Compendium of Policies, Guidelines and Procedures)
This option would be primarily limited to revised price tests that continue to rely completely on domestic and international price referencing methods. This option was fully explored, and included a stakeholder consultation by the PMPRB in 2016, but was rejected on the grounds that simply updating the guidelines does not address the underlying inadequacies of the existing Regulations. Regulatory reform is needed to obtain all price adjustment information and lessen the current dependence on international price testing through the addition of new factors. Under a modernized regulatory framework, the PMPRB would have a stronger basis from which to modernize its guidelines.
Benefits and costs
The quantitative benefits from the cost-benefit statement relate to lower overall spending on patented medicines in Canada that is anticipated to result from lower prices. The quantified costs relate to (1) reduced industry revenues due to lower prices for patented medicines; (2) the net impact of new and reduced administrative industry reporting requirements; and (3) the costs to the Canadian government to ensure compliance with the proposed amendments.
The total quantified benefit of lower patented medicine prices is estimated at $21.3 billion (PV) over 10 years. The total quantified cost of this proposal, including all of the industry's lost revenues, is estimated at $8.6 billion (PV) over 10 years. Administrative costs to industry and the Government of Canada are anticipated to be approximately $62 million (PV) over 10 years. The total net benefits of the proposed amendments are estimated to be $12.7 billion (NPV) over 10 years, from 2019 to 2028. A discount rate of 7% was used in all PV calculations. The complete cost-benefit analysis is available upon request.
Cost-benefit statement
| Base Year (Year 1) |
Final Year (Year 10) |
Total (PV) |
Annualized Average |
|
|---|---|---|---|---|
| Benefits | ||||
| Lower drug expenditure | $219,993,857 | $2,782,694,694 | $8,567,004,599 | $1,219,745,515 |
|
$33,443,984 | $1,399,184,431 | $3,763,190,611 | $535,792,273 |
|
$138,187,981 | $770,272,294 | $2,788,004,256 | $396,948,040 |
|
$48,361,892 | $613,237,969 | $2,015,809,732 | $287,005,201 |
| Health care system | $425,688,113 | $5,384,514,233 | $12,722,001,829 | $1,811,322,089 |
| Total benefits | $645,681,970 | $8,167,208,927 | $21,289,006,428 | $3,031,067,604 |
| Costs | ||||
| Industry | $8,567,068,356 | $1,219,754,583 | ||
|
$219,993,857 | $2,782,694,694 | $8,567,004,599 | $1,219,745,515 |
|
$34,717 | $4,924 | ||
|
$29,106 | $4,144 | ||
| Government | $4,981,481 | $8,025,361 | $61,716,822 | $8,787,064 |
|
$3,849,215 | $5,680,633 | $43,361,629 | $6,173,704 |
|
$981,481 | $2,025,361 | $16,119,394 | $2,295,033 |
|
$143,085 | $304,667 | $2,131,142 | $303,425 |
|
$7,700 | $14,700 | $104,657 | $14,900 |
| Total costs (PV) | $8,628,785,178 | $1,228,541,647 | ||
| Net benefits (NPV) | $12,660,221,250 | $1,802,525,957 | ||
| Qualitative impacts | ||||
|
||||
Costs
Patentee price information reporting requirements already exist under the current regulatory framework. For the most part, the types of information to be reported and the reporting frequency would remain unchanged. The increased administrative burden on the industry would be to report in relation to the new price regulatory factors. The proposal also includes the benefit of reduced administrative burden for certain types of medicines (patented over-the-counter, veterinary, and ANDS-approved medicines), but this reduction would not be sufficient to fully offset the new reporting requirements.
Industry
Industry costs would include the
- Reporting requirements on the new price regulatory factors. Patentees would ensure that the information be updated as new analyses are undertaken. Total administrative costs to report in relation to the new price regulatory factors are estimated to be $6,175 annually or $43,373 in PV over 10 years.
- Compliance cost to update reporting systems to include the proposed schedule of countries on which patentees must report pricing information every six months, and updating their domestic prices and net revenues to include all price adjustments. Patentees already have reporting systems in place for domestic and international prices — the proposal only modifies the type of information to be reported. Total compliance costs are estimated to be $4,144 annually or $29,106 in PV over 10 years.
Administrative burden reduction
The proposal removes the need for patented veterinary, over-the-counter, and generic drugs to file identity and price information with the PMPRB, unless that information is requested by the PMPRB. There are 96 medicine products (out of PMPRB's 1 359) that fall into these categories and are currently required to file information with the PMPRB. Given that the Federal Court of Appeal only recently clarified and upheld the PMPRB's jurisdiction over these medicines, the compliance for reporting of these medicines has not historically been considered by the PMPRB. Assuming full compliance, the administrative burden reduction is expected to be $8,656 (PV) over 10 years.
Lost revenues to the medicine industry
The PMPRB only regulates excessive patented medicine prices in Canada. Any price reduction and repayment of excess revenues that would occur as a result of this proposal would be pursuant to a voluntary compliance undertaking (VCU) by the patentee to comply with the new maximum compliant price levels, or pursuant to a Board Order made following a public hearing before the Board where a Board Panel determines that the medicine has been sold at an excessive price. It is estimated that this proposal will result in reduced industry revenues of approximately $8.6 billion (PV) over 10 years, due to reduced thresholds for maximum non-excessive prices in Canada. For the purpose of this cost-benefit analysis (CBA), national treatment of revenue was given to all patented medicine manufacturers in Canada, despite the fact that 90% of the companies that report to the PMPRB are multinational enterprises (MNEs).
Government of Canada
Increasing the PMPRB's capacity
Costs to Government would include funds for the PMPRB to hire additional staff to support the expected increase in enforcement-related activities, and to administer the new price regulatory factors. The base (2018–19), second (2019–20), third (2020–21), and fourth years (2021–22) would be anticipated to cost $3.8 million, $5.7 million, $6.7 million, and $7.7 million, respectively. From the fifth year onwards, it is anticipated that costs to Government would be $5.7 million/year to maintain the PMPRB's increased capacity.
Increasing special purpose allotment funding
With the proposed new Regulations in place, patentees might be less willing to offer voluntary compliance undertakings and instead press for formal and potentially prolonged hearings. The PMPRB would require additional funding for its special purpose allotment (SPA) to cover the costs of outside legal counsel and expert witnesses. Patentees might also more frequently challenge decisions made under the new regime in the Federal Court. The base (2018–19), second (2019–20), third (2020–21), and fourth years (2021–22) would be anticipated to cost $1.0 million, $1.8 million, $2.8 million, and $3.8 million, respectively. From the fifth year onwards, it is anticipated that costs to Government would be $2.0 million/year to maintain the PMPRB's increased SPA funding.
Offsetting costs to Public Service and Procurement Canada and Shared Services Canada
Increasing the PMPRB's staffing levels would also increase accommodation and information technology (IT) costs. Combined, the base (2018–19), second (2019–20), third (2020–21), and fourth years (2021–22) would be anticipated to cost $151,000, $305,000, $328,000, and $331,000, respectively. From the fifth year onwards, it would be anticipated that costs to Government would be $319,000/year to offset Public Service and Procurement Canada's accommodation costs and Shared Services Canada's IT services costs.
The total cost to the Government of Canada would be anticipated at $61.7 million in net present value over 10 years.
Benefits
Benefits were calculated based on the expected reduction in the level of public risk of excessively priced patented medicines in Canada.
Anticipated quantitative benefits were calculated on the basis of reduced overall spending on patented medicines. The projected baseline of future spending (2017–2028) was calculated using current growth trends and anticipated launches from the current medicine pipeline. It also includes the expected loss of patent protection of medicines that are currently under the PMPRB's jurisdiction. The total net benefits arising from the proposed amendments are estimated to be $25.1 billion dollars (NPV) over 10 years.
Lower patented medicine expenditure
The proposed amendments are expected to lower patented medicine expenditure by an estimated $8.6 billion (PV) over 10 years.
The introduction of the new price regulatory factors would be expected to have the biggest impact on patented medicine expenditure ($3.8 billion), followed by the revised schedule ($2.8 billion) and the reporting of price and sales adjustment with third parties ($2.0 billion).
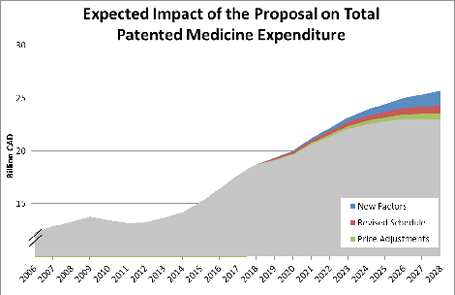
Healthcare system benefits
Without the proposed amendments, it is estimated that public health care systems from across Canada will spend an additional $3.9 billion (PV) for the same quantity of patented medicine. This represents a significant opportunity cost for the Canadian public health care system, as these funds could have been used in other areas of the health care system to better the health of Canadians. Given the large ripple effects on health and the economy for every dollar spent in public health, (see footnote 1) the size of this opportunity cost in Canada is quite substantial. The total opportunity cost to the health care system of paying for excessively priced medicines was estimated to be $12.7 billion dollars (PV) over 10 years.
Sensitivity analysis summary
A sensitivity analysis was performed in relation to two variables that could greatly affect the estimated impact of the proposal. The first variable relates to the PMPRB implementation of the proposal and the other to the projected growth rate in patented medicine expenditure. The baseline analysis was conducted on an assumption that the PMPRB continues to apply price test methods that are similar to those currently in place. This assumption is necessary since any changes to the guidelines are fully within the control of the PMPRB. For example, the PMPRB currently uses the median PMPRB7 price to test new medicines against prices in other countries. The baseline assumes that the median price test would also be applied to the new PMPRB12. The sensitivity analysis of this variable examined possible alternate approaches to the existing price regulatory factors as well as possible approaches to implementation of the proposed new factors in the guidelines.
The second variable relates to the growth of expenditures in patented medicines. If growth in patented medicine expenditure is higher than anticipated, the benefit measured in dollars, calculated from a percent reduction due to lower patented medicine prices, will be higher than anticipated. Likewise, if growth in expenditure is lower than anticipated, then the overall benefit will also be lower. Growth in the patented medicine industry is difficult to predict, and the emergence of new types of patented medicines, such as biologics, introduces new uncertainties into modelling efforts.
The sensitivity analysis demonstrates that total patented medicine expenditure could be lowered from a minimum of $6.4 billion dollars (PV) after 10 years to a maximum of $24.9 billion dollars (PV) after 10 years. The minimum sensitivity analysis impact represents the lowest projected patented medicine sales growth coupled with the least aggressive reforms to the PMPRB guidelines. The maximum sensitivity analysis impact represents the highest projected patented medicine sales coupled with the most aggressive reforms to the PMPRB guidelines. The current CBA estimates the baseline cumulative expenditure after 10 years to be $8.6 billion dollars (PV). (see footnote 2)
Distributional analysis summary
The vast majority of patented medicine manufacturers are located in Ontario, Quebec, British Columbia, and Alberta. These four provinces constitute 98% of all companies that would be affected by the proposed amendments.
All — public, private, and out-of-pocket — payers of patented medicines from across the country will benefit from lower prices.
Usage by age and gender: According to Statistics Canada's report “Prescription medication use by Canadians aged 6 to 79,” prescription medicine use rose with age from 12% among 6- to 14-year-olds to 83% among 65- to 79-year-olds. Prescription medicine use was also associated with the presence of physical and mental health conditions. The percentage of Canadians taking prescription medicines did not differ by household income. Females were generally more likely than males to report taking prescription medications (47% versus 34%). However, at ages 6 to 14, a higher percentage of boys, rather than girls, used prescription medications, and at ages 65 to 79, the prevalence of prescription drug use was similar for men and women. Prescription drug use intensity — the number of different medications taken — was strongly associated with age. The percentage taking more than one medication rose from 3% at ages 6 to 14 to 70% at ages 65 to 79.
“One-for-One” Rule
The estimated added regulatory burden to patentees was calculated to be approximately $43,373, with an estimated reduction in regulatory burden of $8,656, for a total of $34,717 (PV over 10 years). This calculation includes the upfront cost of providing the PMPRB with cost-utility and market size analyses for medicines currently under the jurisdiction of the PMPRB, the ongoing costs of updating these analyses and providing the PMPRB cost-utility analyses and market size estimates for all new patented medicines that enter the market, as well as further reducing the current reporting requirements for patented veterinary, over-the-counter medicines, and adding generic medicines to those same reduced reporting obligations. The proposal is considered an “IN” under the “One-for-One” Rule and has an estimated impact of $3,062.
| Current initiative is an: | “IN” (“One-for-One” Rule) | ||
|---|---|---|---|
| Values to Report in Regulatory Impact Analysis Statement | Rounding | Unit of Measure | |
| Annualized administrative costs (constant $2012) | $3,062 | 0 digits | Constant 2012 dollars, present value base year: 2012 |
| Annualized administrative costs per business ($2012) | $40 | 0 digits | Constant 2012 dollars, present value base year: 2012 |
Small business lens
The small business lens does not apply to the proposed amendments, as only medicine manufacturers that have a patented medicine for sale in Canada would be affected by the proposed amendments. Among the 77 companies reporting to the PMPRB, none were identified as satisfying the small business definition. In general, patented medicines are sold by multinational enterprises or their subsidiaries.
Consultation
The consultation period for prepublication in the Canada Gazette, Part I, of the regulatory proposal will be 75 days.
This consultation builds on an initial consultation on the regulatory proposal. On May 16, 2017, the Honourable Jane Philpott, former federal Minister of Health, announced the launch of the consultation on the proposed amendments to the Patented Medicines Regulations. A consultation document entitled “Protecting Canadians from Excessive Drug Prices: Consulting on Proposed Amendments to the Patented Medicines Regulations” was posted on Health Canada's website as well as the Government of Canada's Consulting with Canadians website. The consultation was promoted through a news release and an email notification that was distributed widely to stakeholders. In addition, to comply with subsection 101(2) of the Patent Act, Minister Philpott wrote each of her counterparts in the provinces and territories, inviting comments on the proposed regulatory amendments. Written submissions from all stakeholders and interested parties were accepted until June 28, 2017. During the consultation period, Health Canada hosted nine engagement sessions with external stakeholders, including representatives from public and private insurers, patient organizations, the medicine industry, the health professions and academia.
Insurers (public and private) were supportive overall, noting that pharmacoeconomic value and market size are very relevant to the determination of price excessiveness. There was no consensus around GDP as a factor. Private insurers suggested that the factors account for considerations relevant to employers, such as the impact of the medicine on productivity, absenteeism, and disability claims. Insurers supported the revised schedule of countries. While in favour of reducing regulatory burden for patented generic medicines, insurers suggested that the PMPRB still request price and sales information for patented generics at risk of higher prices. Finally, insurers were supportive of the amendment to provide the PMPRB with price adjustment information, on the condition that this information remain confidential to the PMPRB.
Patient organizations noted that the high prices of new patented medicines pose a financial barrier to access for Canadians and asked that the Regulations ensure that patient access to medicines is a primary concern. Patient organizations suggested that there be enough flexibility in the Regulations to allow the PMPRB to go beyond the cost per QALY to take patient preferences into account and to consider special circumstances such as medicines for rare diseases. In addition, organizations asked that the use of price adjustment information in regulating prices not compromise the bargaining position of insurers.
Representatives of the brand name medicine industry suggested that proposed amendments would add significant complexity and uncertainty for patented medicines to reach the market in Canada. A number of representatives suggested that the proposed economic-based factors go beyond the mandate of the PMPRB and are potentially duplicative of CADTH's assessment. They expressed concern around the additional regulatory burden of providing international pharmacoeconomic and pricing information. A common suggestion was that the United States should remain in the schedule of countries. It was recommended that the Regulations allow for a risk-based approach and that regular reporting requirements should be removed for lower-risk products. It was not clear to the industry how the PMPRB plans to use and protect confidential price adjustment information; however, it was suggested that providing this information to the PMPRB would risk lower price adjustments for insurers in Canada.
Generic medicine industry representatives supported the proposal to remove the requirement for patented generic manufacturers to regularly report information about the identity and price of these medicines, as they pose a low risk of abusing market power and are subject to price regulation by the provinces and territories. They recommended this amendment be extended to include other complex forms of generics that do not receive a Declaration of Equivalence from Health Canada, such as biosimilars and generics with complex ingredients and formulations.
The consumer health products industry acknowledged that the over-the-counter products (OTCs) it produces are already exempt from reporting regularly. Representatives recommended that all self-care products be exempt entirely from the patented medicine framework; however, it is beyond the scope of the Regulations to change the PMPRB's jurisdiction over patented medicines.
Representatives from physicians' and nurses' associations supported economics-based factors to assess the value of a medicine, the revised schedule and requiring information on confidential rebates in Canada. Nurses' associations were not supportive of exempting patented generics from systematic reporting requirements. Pharmacists supported assessing a medicine based on its value, but noted that pharmacoeconomic value should consider benefits and costs beyond a QALY. They noted that the schedule of comparator countries should be revised based on the availability of products in each country and asked that the amendment pertaining to confidential price adjustments not compromise the price adjustments negotiated by public insurers.
Academics supported the proposed pharmacoeconomic value factor and cost per QALY information requirement. Some academics supported using GDP to set an upper bound on prices and suggested the use of per capita GDP. Academics were less convinced that market size information would be useful without more information on the R&D costs of a medicine. Most agreed with revising the schedule and removing countries that do not have consumer protections in place for excessive prices. Academics were generally in favour of allowing the PMPRB to collect information on adjustments in price, but they suggested it be broadened to include all types of transfers from patentees that impact prices, including pay-for-performance agreements, and cautioned against using rebate information when making international comparisons.
The responses related to the Regulations have been taken into consideration in the development of this proposal for prepublication in the Canada Gazette, Part I, and the Regulatory Impact Analysis Statement. In particular,
- The economics-based price regulatory factors in the proposed amendments have remained broad in order to provide the PMPRB with the flexibility to consider other measures beyond the cost per QALY where relevant, and to enable the PMPRB to develop appropriate measures using market size and GDP. Based on feedback received, GDP per capita has been added to the GDP factor.
- The information reporting requirements for patentees have been revised to minimize the regulatory burden while providing the PMPRB with sufficient information to protect Canadians from excessive prices. The proposed amendments do not require cost-utility analyses (CUAs) from countries other than Canada to be reported.
- Further analysis has been provided on the proposed schedule; an estimate of the impacts on patented medicine expenditures is provided in the cost-benefit analysis.
- Consideration was given to the removal of systematic information reporting requirements for patentees for other low-risk products beyond patented generic medicines. It is proposed that regular reporting requirements be removed for all patented over-the-counter medicines, including radiopharmaceuticals and biologics authorized for sale under the Food and Drug Regulations as well as those containing controlled substances. While other products such as biosimilars and other patented generic medicines that are not authorized for sale by way of an ANDS were considered, these products and their risk of excessive pricing could not be adequately defined.
- It is proposed that the new information reporting requirements in the Regulations capture all price adjustments that would serve to lower (e.g. discounts, rebates, free goods, free services) or raise (e.g. payment for performance) the price of a medicine.
Regulatory cooperation
This proposal would update the schedule of countries used by the PMPRB for international price comparisons to be better aligned with the PMPRB's consumer protection mandate and median OECD prices. This international alignment would contribute to lowering medicine prices for Canadians.
Rationale
Unlike most international health systems, Canada's health system does not have a single payer for medicines. Canadian expenditure on prescription medicines is split between public insurers (43%), private insurers (35%) and Canadians paying out-of-pocket (22%).
Modernization of the PMPRB's regulatory framework would benefit all those who pay for medicines in Canada through a higher standard of consumer protection. Canada's public and private insurers would benefit from lower maximum prices so their price negotiations achieve more than simply prices that match those in other countries. The amendments would help the PMPRB to achieve Canadian maximum prices closer to international norms. This would allow public and private insurers to negotiate with sellers on a more equal footing with health authorities in other countries. Employer-sponsored health insurance plans are anticipated to benefit from lower premiums and reduced risk of becoming untenable due to high-cost medicines. Uninsured Canadians who pay out-of-pocket for their medicines rely most heavily on the consumer protection mandate of the PMPRB, and they would benefit from lower prices for their patented medicines.
This proposal is anticipated to result in an estimated total benefit to Canadians of $8.6 billion in net present value (NPV) over 10 years following implementation.
Implementation, enforcement and service standards
The proposed Regulations would come into force on January 1, 2019. This would allow patentees time to prepare for implementation of the new price regulatory factors and information reporting requirements on prices. January 1, 2019, was the date chosen to align the implementation with the PMPRB's reporting periods of January 1 and July 1. Once the amended Regulations are published in the Canada Gazette, Part II, responsibility for implementation, enforcement and service standards would be passed to the PMPRB. This is anticipated to include the finalization of a PMPRB-led stakeholder consultation on a revised Compendium of Policies, Guidelines and Procedures that will be used to reach an understanding of how the revised framework would be embodied in the form of specific price tests and qualifying information to be reported by patentees.
The new factors may only be considered in relation to sales that occur after the coming into force of the proposed amendments. However, the reporting requirements in the amended Regulations would be applied to new and existing patented medicines alike. Patentees of existing medicines would have 30 days after the coming into force to provide the cost-utility analysis (if available) and estimated market use information (if applicable). Price information for the countries in the revised schedule and domestic price and revenue information that takes into account price adjustments would first be required to be reported within 30 days after the end of the reporting period in which the proposed amendments came into force (i.e. within 30 days after June 30, 2019).
Contact
Karen Reynolds
Executive Director
Office of Pharmaceuticals Management Strategies
Strategic Policy Branch
Health Canada
Brooke Claxton Building, 10th Floor
70 Colombine Driveway, Tunney's Pasture
Ottawa, Ontario
K1A 0K9
Telephone: 613-957-1692
Email: PMR-Consultations-RMB@hc-sc.gc.ca
PROPOSED REGULATORY TEXT
Notice is given that the Governor in Council, pursuant to subsection 101(1) (see footnote a) of the Patent Act (see footnote b), proposes to make the annexed Regulations Amending the Patented Medicines Regulations.
Interested persons may make representations concerning the proposed Regulations within 75 days after the date of publication of this notice. All such representations must cite the Canada Gazette, Part I, and the date of publication of this notice, and be addressed to Karen Reynolds, Executive Director, Office of Pharmaceuticals Management Strategies, Strategic Policy Branch, Health Canada, 10th Floor, Brooke Claxton Building, 70 Colombine Driveway, Tunney's Pasture, Ottawa, Ontario K1A 0K9 (tel.: 613-957-1692; email: PMR-Consultations-RMB@hc-sc.gc.ca).
Ottawa, November 23, 2017
Jurica Čapkun
Assistant Clerk of the Privy Council
Regulations Amending the Patented Medicines Regulations
Amendments
1 Section 3 of the Patented Medicines Regulations (see footnote 3) is amended by adding the following after subsection (3):
(3.1) Despite subsection (3), in each of the following cases, the information referred to in subsection (1) must be provided to the Board within 30 days after the day on which the Board sends a request for the patentee to provide that information:
- (a) the medicine is not a prescription drug as defined in section A.01.010 of the Food and Drug Regulations;
- (b) the medicine contains a controlled substance as defined in subsection 2(1) of the Controlled Drugs and Substances Act, the sale or provision of which does not require a prescription under that Act;
- (c) a notice of compliance has been issued in respect of the medicine on the basis of information and material contained in a submission filed under section C.08.002.1 of the Food and Drug Regulations; or
- (d) the medicine is for veterinary use.
2 (1) The portion of subsection 4(2) of the Regulations before paragraph (a) is replaced by the following:
(2) The information referred to in subsection (1) must be provided
(2) Subsection 4(3) of the Regulations is replaced by the following:
(3) Despite subsection (2), in each of the following cases, the information referred to in subsection (1), for each six-month period beginning on January 1 and July 1 of each year, must be provided to the Board within 30 days after the day on which the Board sends a request for the patentee to provide that information and, during the two years following the request, within 30 days after the end of each six-month period:
- (a) the medicine is not a prescription drug as defined in section A.01.010 of the Food and Drug Regulations;
- (b) the medicine contains a controlled substance as defined in subsection 2(1) of the Controlled Drugs and Substances Act, the sale or provision of which does not require a prescription under that Act;
- (c) a notice of compliance has been issued in respect of the medicine on the basis of information and material contained in a submission filed under section C.08.002.1 of the Food and Drug Regulations; or
- (d) the medicine is for veterinary use.
(3) Paragraphs 4(4)(a) and (b) of the Regulations are replaced by the following:
- (a) in calculating the average price per package of a medicine, the actual price obtained by the patentee must be used, taking into account any adjustments that are made by the patentee or any party that directly or indirectly purchases or reimburses for the purchase of the medicine and any reduction given to any party in the form of free goods, free services, gifts or any other benefit of a like nature; and
- (b) in calculating the net revenue from sales of each dosage form, strength and package size in which the medicine was sold in final dosage form, the actual revenue obtained by the patentee must be used, taking into account any adjustments that are made by the patentee or any party that directly or indirectly purchases or reimburses for the purchase of the medicine and any reduction given to any party in the form of free goods, free services, gifts or any other benefit of a like nature.
3 The Regulations are amended by adding the following after section 4:
4.1 (1) For the purposes of paragraphs 80(1)(d) and (2)(d) of the Act, the information to be provided respecting the factor referred to in paragraph 4.4(a) is every cost-utility analysis prepared by a publicly funded Canadian organization, if published, for which the outcomes are expressed as the cost per quality-adjusted life year for each indication that is the subject of the analysis.
(2) The information referred to in subsection (1) must be provided
- (a) if the information is published when the medicine is first offered for sale in Canada, within 30 days after the day on which the medicine is first offered for sale in Canada; and
- (b) if the information is not published when the medicine is first offered for sale in Canada, within 30 days after the day on which it is published.
(3) Despite subsection (2), in the case of a medicine that is offered for sale in Canada before January 1, 2019, the information referred to in subsection (1) must be provided
- (a) if the information is published before January 1, 2019, by January 30, 2019; and
- (b) if the information is not published before January 1, 2019, within 30 days after the day on which it is published.
(4) If any other analysis as described in subsection (1) is published after those referred to in subsection (1) were provided, it must be provided within 30 days after the day on which it is published.
4.2 (1) For the purposes of paragraphs 80(1)(d) and (2)(d) of the Act, the information to be provided respecting the factor referred to in paragraph 4.4(b) is the estimated maximum use of the medicine in Canada, by quantity of the medicine in final dosage form, for each dosage form and strength that are expected to be sold.
(2) The information referred to in subsection (1) must be provided within 30 days after the day on which the medicine is first offered for sale in Canada.
(3) Despite subsection (2), in the case of a medicine that is offered for sale in Canada before January 1, 2019, the most recent version of the information referred to in subsection (1) must be provided
- (a) if the medicine is first offered for sale in Canada during the period beginning on January 1, 2016 and ending on December 31, 2018, by January 30, 2019; and
- (b) if the information referred to in subsection (1) in respect of the medicine is not required to be provided under paragraph (a), but the information is updated
- (i) during the period beginning on January 1, 2016 and ending on December 31, 2018, by January 30, 2019; or
- (ii) after December 31, 2018, within 30 days after the day on which it is updated.
(4) The information provided under this section must be up to date and any modification of that information must be provided within 30 days after the day on which the modification is made.
4.3 (1) Despite subsections 4.1(2) and (3) and 4.2(2) and (3), in each of the following cases, the information referred to in subsections 4.1(1) and 4.2(1) must be provided to the Board within 30 days after the day on which the Board sends a request for the patentee to provide that information:
- (a) the medicine is not a prescription drug as defined in section A.01.010 of the Food and Drug Regulations;
- (b) the medicine contains a controlled substance as defined in subsection 2(1) of the Controlled Drugs and Substances Act, the sale or provision of which does not require a prescription under that Act;
- (c) a notice of compliance has been issued in respect of the medicine on the basis of information and material contained in a submission filed under section C.08.002.1 of the Food and Drug Regulations; or
- (d) the medicine is for veterinary use.
(2) The requirements of subsections 4.1(4) and 4.2(4) apply in respect of the information provided under subsection (1).
Other Factors to be Considered — Excessive Prices
4.4 For the purposes of paragraph 85(1)(e) of the Act, the other factors that the Board must take into consideration to determine whether a medicine that is sold in any market in Canada after December 31, 2018 is being or has been sold at an excessive price are the following:
- (a) the pharmacoeconomic value in Canada of the medicine and that of other medicines in the same therapeutic class;
- (b) the size of the market for the medicine in Canada and in countries other than Canada; and
- (c) the gross domestic product in Canada and the gross domestic product per capita in Canada.
4 The schedule to the Regulations is replaced by the schedule set out in the schedule to these Regulations.
Coming into Force
5 These Regulations come into force on January 1, 2019.
SCHEDULE
(Section 4)
SCHEDULE
(Subparagraph 4(1)(f)(iii))
- Australia
Australie - Belgium
Belgique - France
France - Germany
Allemagne - Italy
Italie - Japan
Japon - Netherlands
Pays-Bas - Norway
Norvège - Republic of Korea
République de Corée - Spain
Espagne - Sweden
Suède - United Kingdom
Royaume-Uni
[48-1-o]