Canada Gazette, Part I, Volume 152, Number 51: Regulations Amending the Cannabis Regulations (New Classes of Cannabis)
December 22, 2018
Statutory authority
Cannabis Act
Sponsoring department
Department of Health
REGULATORY IMPACT ANALYSIS STATEMENT
(This statement is not part of the Regulations or the Order.)
Executive summary
Issues: The Cannabis Act will authorize the legal sale of "edibles containing cannabis" and "cannabis concentrates" one year following the coming into force of the Act. Amendments to the Cannabis Regulations are required to address the public health and public safety risks of these new classes of cannabis, and must be brought into force by no later than October 17, 2019.
Description: It is proposed to amend Schedule 4 to the Cannabis Act to add three new classes of cannabis that could be legally sold by federal licence holders and provincially and territorially authorized distributors and retailers. Those three new classes would be "edible cannabis," "cannabis extracts," and "cannabis topicals."
Consistent with the comprehensive public health approach to the regulation of all cannabis products established by the Cannabis Regulations, it is also proposed to amend the Cannabis Regulations to establish new regulatory controls to address the public health and public safety risks associated with these new classes of cannabis, including their appeal to youth and the risks of accidental consumption, overconsumption, and foodborne illness, among other risks. These controls would include restrictions on product composition and ingredients, tetrahydrocannabinol (THC) limits, and new requirements pertaining to packaging and labelling, good production practices and record keeping.
At the same time, it is proposed to amend the Cannabis Regulations to allow for a broader variety of product forms within these new classes of cannabis, consistent with the Government's objective of displacing the illegal market.
Rationale: The proposed amendments to the Cannabis Regulations are estimated to generate a net cost to federal licence holders of $40.5 million net present value over a 10-year period (or $5.8 million annually). In contrast, the public health and public safety benefits resulting from the current proposal are considerable, even if they cannot be quantified. It is expected that these benefits would outweigh the costs. As required by the "One-for-One" Rule, the incremental increase in administrative burden for industry resulting from the proposed amendments has been estimated at $352,876 net present value over the 10-year period (or $50,242 annually). This increase in administrative burden is mostly related to the proposed new record-keeping requirements.
The current proposal is subject to a public comment period ending 60 days following its publication in the Canada Gazette, Part I. All input received through the public consultation will inform development of the final regulations.
Given the provincial and territorial responsibility for distribution and retail sale of cannabis, Health Canada will hold bilateral meetings with representatives of all provincial and territorial governments to help ensure their perspectives and feedback are considered in the development of final regulations.
Engagement with Indigenous peoples has been ongoing throughout the development of the Cannabis Act and its regulations. Health Canada welcomes the views of Indigenous peoples with respect to the new classes of cannabis and the current proposal, and will continue to engage and work closely with Indigenous governments, organizations and communities across the country to help ensure the specific interests of Indigenous peoples are carefully considered.
Issues
The Cannabis Act (the Act) will authorize the legal sale of "edibles containing cannabis" and "cannabis concentrates" one year following the coming into force of the Act (i.e. on October 17, 2019), unless section 193.1 of the Act footnote 1 is brought into force sooner, by Order. Amendments to the Cannabis Regulations (the Regulations) are required to address the public health and public safety risks of these new classes of cannabis, and must be brought into force by no later than October 17, 2019.
Consistent with the comprehensive public health approach to the regulation of all cannabis products established by the Regulations, a series of targeted amendments to the Regulations are proposed to address the public health and public safety risks associated with edible cannabis and cannabis products with concentrated levels of phytocannabinoids. At the same time, the current proposal would enable a comprehensive range of cannabis product forms by regulating three new product classes, specifically "edible cannabis," "cannabis extracts," and "cannabis topicals."
Background
In the 2015 Speech from the Throne, the Government of Canada committed to legalizing, strictly regulating, and restricting access to cannabis. In June 2016, the Task Force on Cannabis Legalization and Regulation (the Task Force) was established. Composed of nine distinguished experts in public health, substance use, law enforcement and justice, the Task Force was mandated to consult broadly with Canadians and to provide advice on the design of a new legislative and regulatory framework. The Task Force consulted extensively on a detailed discussion paper with provinces and territories, Indigenous governments and organizations and experts in relevant fields, including public health, substance use, criminal justice, law enforcement and industry, as well as with youth.
The Task Force received more than 30 000 responses to its public consultation and more than 300 written submissions from organizations or individuals. It delivered its final report, A Framework for the Legalization and Regulation of Cannabis in Canada, on December 13, 2016. In it, the Task Force made 85 recommendations for the establishment of a comprehensive framework for the legalization and regulation of cannabis across five themes: minimizing harms of use; establishing a safe and responsible supply chain; enforcing public safety and protection; medical access; and implementation.
On April 13, 2017, the Government of Canada introduced Bill C-45 (the Cannabis Act) in the House of Commons. Based in large part on the advice provided by the Task Force, the Cannabis Act created the foundation for a comprehensive national framework to provide restricted access to regulated cannabis, and to control its production, distribution, sale, importation, exportation, and possession. Following Parliamentary study, the Cannabis Act received royal assent on June 21, 2018, and was brought into force on October 17, 2018.
As set out in section 7, the purpose of the Act is to protect public health and public safety and in particular to
- Protect the health of young persons by restricting their access to cannabis;
- Protect young persons and others from inducements to use cannabis;
- Provide for the legal production of cannabis to reduce illegal activities in relation to cannabis;
- Deter illegal activities in relation to cannabis through appropriate sanctions and enforcement measures;
- Reduce the burden on the criminal justice system in relation to cannabis;
- Provide access to a quality-controlled supply of cannabis; and
- Enhance public awareness of the health risks associated with cannabis use.
The Cannabis Regulations, which also came into force on October 17, 2018, established the rules and standards that apply to the authorized production, distribution, sale, importation and exportation of cannabis (with the exception of industrial hemp), as well as other related activities respecting the five classes of cannabis (i.e. dried cannabis, fresh cannabis, cannabis oil, cannabis plants, and cannabis plant seeds) that can be sold by authorized persons.
The Cannabis Regulations set out a comprehensive public health approach to regulating the production, packaging and labelling of all classes of cannabis. More specifically, under the Regulations
- Licences are required in order to cultivate or process cannabis, to sell cannabis for medical purposes, to manufacture prescription drugs containing cannabis, or to conduct analytical testing of or research with cannabis;
- Licence holders are subject to strict physical and personnel security requirements;
- The production of cannabis products by licence holders is subject to strict rules and standards, including limits on tetrahydrocannabinol (THC) content and the use of additives, as well as good production practices;
- Plain packaging and labelling is required for cannabis products, including strict limits on the use of logos, colours, and branding; and
- Continued access to cannabis for medical purposes is provided for patients who need it.
Further information on the Regulations is available in the Regulatory Impact Analysis Statement that was published in the Canada Gazette, Part II, on July 11, 2018.
Objective
Building on the framework established by the current Regulations, the objective of this regulatory proposal is to ensure that a strict regulatory framework is in place by no later than October 17, 2019, to address the unique public health and public safety risks posed by edible cannabis, cannabis extracts, and cannabis topicals.
In particular, the current proposal aims to protect public health and public safety by reducing the
- Appeal of such products to youth;
- Risk of accidental consumption, especially of edible cannabis, including by youth;
- Risk of overconsumption associated with edible cannabis (because of the delay in experiencing the effects of cannabis when it is ingested rather than inhaled) and cannabis products with a higher concentration of THC;
- Risk of foodborne illness associated with the production and consumption of edible cannabis;
- Risk of dependence and other negative health outcomes associated with cannabis products with a higher concentration of THC or cannabis products that contain ethyl alcohol or caffeine; and
- Potential health and, in some cases, safety risks associated with the use of certain solvents, carriers, and diluents.
At the same time, consistent with the objective as set out in section 7 of the Act to provide for the legal production of cannabis to reduce illegal activities in relation to cannabis, the current regulatory proposal aims to enable a comprehensive range of cannabis products by adding three new classes of cannabis to Schedule 4, and by making targeted amendments to the Regulations to allow for a broader variety of product forms within these new classes of cannabis.
Finally, a number of minor amendments to the Regulations are proposed to clarify the original policy intent behind the provisions being amended or to facilitate compliance with the regulatory requirements.
Description
The development of the current proposal, as well as the Cannabis Regulations, has been informed by
- Consultations conducted by the Task Force on Cannabis Legalization and Regulation;
- The experience of U.S. jurisdictions that have legalized and regulated access to cannabis;
- Parliamentary study of Bill C-45;
- Consultations supporting the development of the Cannabis Regulations; and
- Ongoing feedback from regulated parties and other stakeholders.
The current proposal has two main parts:
- It is proposed that Schedule 4 to the Cannabis Act be amended by Order in Council to add three new classes of cannabis that could be sold by authorized persons, namely "edible cannabis," "cannabis extracts," and "cannabis topicals."
- It is proposed that the Cannabis Regulations be amended to establish new regulatory controls to address the public health and public safety risks associated with these new classes of cannabis.
The proposed amendments to the Regulations focus on those necessary to regulate the new classes of cannabis, and are based on the following policy principles:
- New classes of cannabis are integrated into the existing cannabis control framework: Consistent with the Government of Canada's stated public health and public safety objectives, all cannabis products are to be strictly regulated under the Cannabis Act and its regulations.
- Proposed new requirements are evidence-informed: New regulatory requirements are based on the best-available public health information on the risks and harms posed by these new classes of cannabis, as well as relevant experience from U.S. jurisdictions that have legalized and regulated access to cannabis.
- Proposed new requirements are consistent with analogous regulatory frameworks: To the extent that they support the Government's stated public health and public safety objectives, new regulatory requirements draw from, and are consistent with, other relevant control frameworks, including those for food, vaping products and cosmetics.
- Amendments would enable a comprehensive range of product forms: Consistent with the objective of enabling the legal industry to displace the illegal industry, amendments to the Regulations are proposed with a view to enabling a comprehensive range of product forms intended for human use.
Schedule 4: Classes of cannabis that an authorized person may sell
Schedule 4 to the Act sets out the classes of cannabis that can legally be sold by "authorized persons," including federal licence holders and provincially and territorially authorized distributors and retailers. Currently, the following five classes of cannabis are listed on Schedule 4: dried cannabis, cannabis oil, fresh cannabis, cannabis plants, and cannabis plant seeds.
As per section 193.1 and subsection 226(2) of the Act, "edibles containing cannabis" and "cannabis concentrates" must be added to Schedule 4 to the Act by no later than October 17, 2019. The Governor in Council may authorize the legal sale of these product classes in advance of this date, by order.
Therefore, it is proposed that the Governor in Council make an order adding three new classes of cannabis to Schedule 4. These three classes of cannabis would be as follows:
- Edible cannabis: products containing cannabis that are intended to be consumed in the same manner as food (i.e. eaten or drunk);
- Cannabis extracts: products that are produced using extraction processing methods or by synthesizing phytocannabinoids; and
- Cannabis topicals: products that include cannabis as an ingredient and that are intended to be used on external body surfaces (i.e. skin, hair, and nails).
It is also proposed that an order would be made to remove "cannabis oil" from Schedule 4 six months after the amended Regulations come into force, in order to provide for an appropriate transition period. Following the six-month transition period, cannabis oil would be subsumed under the new product classes.
As will be described in greater detail below, while the term "cannabis concentrates" would not be one of the names of the new permitted classes of cannabis listed on Schedule 4, the current proposal would nonetheless permit the legal production and sale of cannabis products with higher concentrations of THC, consistent with the will of Parliament.
Licensing
Part 2 of the Regulations establishes the classes (e.g. cultivation, processing, sale) and subclasses (e.g. standard and micro-cultivation, standard and micro-processing) of licences that authorize activities with cannabis at the federal level, as well as the rules and requirements that apply to each licence class and subclass.
No major amendments to Part 2 are proposed. As is currently the case for dried cannabis, fresh cannabis and cannabis oil, it is proposed that a processing licence (standard or micro) would be required in order to manufacture edible cannabis, cannabis extracts and cannabis topicals, and to package and label these types of cannabis products for sale to consumers.
The following minor amendments are proposed:
- It is proposed that a past conviction for an offence under the Safe Food for Canadians Act (SFCA) or any of the Acts that will be repealed when the SFCA comes into force would be added as a ground for the refusal or revocation of a processing licence, if the conviction occurred within the preceding 10 years.
- Section 19 of the Regulations states that a licensed processor must retain the services of an individual with the necessary training, experience and technical knowledge to act as a quality assurance person (QAP). It is proposed that the Regulations would specify that the QAP would need to have the necessary qualifications to oversee the production of all classes of cannabis that the licensed processor is authorized to produce (including training, experience, and technical knowledge of both good production practices and product rules). In the event that the QAP does not have the requisite knowledge and training with respect to edible cannabis, the licence holder would be required to retain the services of another individual with the necessary qualifications.
- Section 46 of the Regulations requires licence holders to establish and maintain a system of control to support efficient product recalls. Building on that requirement, it is proposed to add a new requirement that licence holders conduct, at least once every 12 months, a recall simulation to evaluate the effectiveness of their recall systems and processes, and that they prepare a document describing how the simulation was conducted and the results. This document would need to be kept for at least two years. This is adapted from a requirement under the Safe Food for Canadians Regulations (SFCR).
Personnel and physical security
Parts 3 and 4 of the Regulations set out requirements pertaining to security clearances and physical security measures, respectively. No amendments are proposed to either part. Licence holders conducting activities with the new classes of cannabis would be subject to the same strict physical and personnel security requirements established under the Regulations.
Good production practices
Part 5 of the Regulations establishes requirements pertaining to the production, distribution and storage of cannabis to control the quality of cannabis produced by federal licence holders (i.e. good production practices). Requirements set out in Part 5 apply to many aspects of the production process, including the equipment being used, the sanitation program, quality assurance, storage and distribution of cannabis products, and standard operating procedures.
It is proposed that Part 5 would be amended to incorporate additional good production practices to prevent contamination of cannabis products and to address the risk of foodborne illness associated with edible cannabis. Many of the new requirements, particularly those pertaining to edible cannabis, are adapted from the SFCR. For example, it is proposed that
- Requirements that pertain to the cleanliness of equipment used with cannabis or ingredients would be expanded to also include conveyances (in this context, the term "conveyance" refers to anything that is used within the licensed facility to transport cannabis or ingredients used in the production of cannabis products; an example would be a forklift or hand lift), consistent with the SFCR (the requirement would apply to both licensed cultivators and licensed processors).
- Building on existing air filtration requirements to prevent the escape of odours, there would be a new requirement to have a ventilation system that provides clean air and removes unclean air that may have a negative impact on the cannabis or ingredients. These measures, which would apply to both licensed cultivators and processors, are intended to prevent contamination, and are consistent with measures under the SFCR.
- Sanitation program requirements would be expanded to explicitly require hand cleaning/sanitizing stations and lavatories in buildings where cannabis is produced (by licensed cultivators and licensed processors), if necessary, to prevent the contamination of cannabis or ingredients. There would also be a new requirement (that would apply to licensed processors only) pertaining to employee clothing, footwear, and protective coverings. Both new requirements are consistent with the SFCR. While current sanitation program requirements under the Regulations do not explicitly include such requirements, it is anticipated that most, if not all, of the new elements already form part of sanitation programs in place at most licensed facilities.
- Existing controls designed to prevent the contamination of cannabis would be expanded to also cover ingredients intended to form part of a cannabis product (licensed processors only).
- Consistent with the SFCR, licensed processors (that produce edible cannabis or cannabis extracts) would be required to prepare, retain, maintain and implement a written preventive control plan (PCP) to identify and address through effective control measures any potential hazards that pose a risk to the production of these products. The QAP would be required to sign-off on the PCP prior to its implementation. Furthermore, the PCP would need to include documents that substantiate that the PCP has been implemented.
- Employees of licensed processors who conduct activities involving edible cannabis (or ingredients used in the production of edible cannabis) would be required to have the necessary competencies and qualifications to carry out their duties, consistent with the SFCR.
- Currently, the QAP is required to investigate every complaint received in respect of the quality of the cannabis and to take measures to address any identified risk. In addition to the current requirement, it is proposed that the QAP would be required to proactively conduct an investigation any time they suspect that cannabis or an ingredient may present a risk of injury to human health or does not meet requirements in Part 5 or Part 6 of the Regulations, and, if necessary, to immediately take measures to mitigate any risk. The proposed new requirement, which is adapted from the SFCR, would apply, for example, in a situation where the QAP suspects that an ingredient may have been improperly stored, resulting in contamination that presents a risk to human health.
- Licensed processors would need to ensure that steps are taken so that animals and pests are not able to enter into any building or part of a building where cannabis is being processed. While this requirement is taken from the SFCR, it would apply to all licensed processors (not just those processing edible cannabis). Although this is not currently a requirement under the Regulations, it is anticipated that most, if not all, of licensed facilities already have such measures in place.
- There would be a requirement that any water (including ice or steam used in the production of a cannabis product) coming into contact with cannabis or an ingredient be potable, unless the water does not present a risk of contamination, consistent with the SFCR. This requirement would apply to licensed processors producing the new classes of cannabis.
A number of measures are proposed to prevent the contamination of cannabis or ingredients. One proposed requirement is that licensed processors would be required to separate incompatible activities and ensure that contaminated waste is disposed of properly. Another measure is that licensed processors producing edible cannabis would be required to identify and place contaminated ingredients in a designated area, also consistent with the SFCR. Furthermore, the requirement (section 80 of the Regulations) to prepare standard operating procedures for the production, packaging, labelling, distribution, storage, sampling, and testing of cannabis would be amended to also pertain to a licence holder's handling of ingredients, and to specify that all such activities must be conducted in accordance with the requirements set out in both Part 5 and Part 6 of the Regulations (currently, section 80 applies only to cannabis, and specifies only that activities must be conducted in accordance with Part 5).
In addition, it is proposed that production of edible cannabis at a site where conventional food products are also being manufactured for sale could only be done if the edible cannabis was being produced within another building within the licensed site. This proposal is intended to mitigate against the food safety and public health concerns associated with multiproduct manufacturing facilities, and in particular to mitigate against the risks of cross-contamination between ingredients and products, and the increased risk of mislabelling and product mix-ups. It also provides Canada's international trade partners or importers of Canadian food products with assurance that there can be absolutely no cross-contamination of Canadian food products with cannabis.
Testing
Part 5 of the Regulations also sets out requirements pertaining to the sampling and testing of cannabis. The current Regulations require that the following testing be conducted on the final form of cannabis products:
- Testing to determine the content of THC, tetrahydrocannabinolic acid (THCA), cannabidiol (CBD), and cannabidiolic acid (CBDA);
- Testing for microbial and chemical contaminants;
- Testing for the residues of solvents used in the production of cannabis oil; and
- Dissolution or disintegration testing (on discrete units intended for ingestion or nasal, rectal, or vaginal use).
The following amendments to the Regulations are proposed:
- While the Regulations currently distinguish between testing for solvent residues and chemical contaminants, solvent residues are a form of chemical contaminant. For this reason, it is proposed that the amended Regulations would treat solvent residues in the same way as other chemical contaminants, such as heavy metals. Solvent residue testing would continue to be required any time a solvent is used in the preparation of a cannabis product.
- It is proposed that the licensed processor, when conducting microbial and chemical contaminant testing (including solvent residue testing), would have the option of conducting testing on either the final form of the cannabis product, or at the final step in the production process during which the contaminants could be concentrated (i.e. on the "input" cannabis). For example, if a cannabis extract is used in the production of a cannabis topical, the licensed processor would have the option of conducting testing on the cannabis extract or on the final form of the cannabis topical.
- Furthermore, whereas currently, levels of microbial and chemical contaminants must be within established limits for herbal medicines, it is proposed that microbial and chemical limits would need to be within the limits that are appropriate for the intended use of the product (e.g. ingestion, inhalation).
Proposed product rules for the new classes of cannabis
Part 6 of the Regulations sets out the rules that apply to the production of cannabis products, by product class. Given that no rules currently exist with respect to the new classes of cannabis, amendments to this part are required in order to establish rules for edible cannabis, cannabis extracts, and cannabis topicals. It is proposed that these would include THC limits per serving and/or per package, and rules pertaining to product composition and ingredients.
THC limits
To reduce the risks associated with overconsumption and accidental consumption, it is proposed that limits would be placed on the amount of THC that could be in the products of the new classes of cannabis, in individual servings (or "discrete units") and in a single package. Specifically, it is proposed that
- For edible cannabis, there would be a limit of 10 milligrams of THC footnote 2 per discrete unit and per package. This would mean, for example, that a package could contain one discrete unit of edible cannabis that contains 10 milligrams of THC; or two discrete units that each contain 5 milligrams of THC.
- For cannabis extracts, as is currently the case for cannabis oil, there would be a limit of 10 milligrams of THC per discrete unit that is intended to be ingested or for nasal, rectal, or vaginal use, such as a capsule. In addition, there would be a new limit of 1 000 milligrams (or 1 gram) of THC in a single package. This would mean, for example, that a package could contain 100 capsules of an extract that each contain 10 milligrams of THC; or 200 capsules of an extract that each contain 5 milligrams of THC.
- For cannabis topicals, there would be a limit of no more than 1 000 milligrams (or 1 gram) of THC in a package.
In addition, a lower possession limit and smaller package sizes would apply to any cannabis product that contains more than 3% THC by weight. Consistent with the Act and current Regulations (paragraph 108(f)), the maximum package size and public possession limit of 7.5 grams (equivalent to 30 grams of dried cannabis) would apply to edible cannabis, cannabis extracts, or cannabis topicals that contain more than 3% w/w THC (i.e. products that are considered "concentrates" for the purposes of Schedule 3 to the Act).
Establishing limits on the amount of THC that could be in the new classes of cannabis is considered a more effective means of addressing the risks of accidental consumption and overconsumption than establishing a maximum concentration of THC (or "potency") that could be in a product.footnote 3
Product composition and ingredients
Currently, the Regulations do not permit the addition of anything other than cannabis to cannabis products (with the exception of cannabis oil, which may only contain the carrier oil and any additives necessary to preserve the quality and stability of the product). Consistent with the objective of enabling the legal cannabis industry to displace the illegal market, targeted amendments to the Regulations are proposed that would permit a broader diversity of product forms for human use.
At the same time, consistent with the comprehensive public health approach to the Regulations, certain limits are important safeguards and would remain in place. For example, product forms that pose a greater risk to human health, such as products that are intended to be used in the area of the human eye (e.g. eye drops) or products that are intended to be used on damaged or broken skin or to penetrate the skin barrier by means other than by absorption (e.g. through the use of abrasives or needles) would continue to be prohibited.
It is proposed that the Regulations would establish the following "variability limits" footnote 4 for the amount of THC and CBD in the new classes of cannabis:
- For edible cannabis, if the total quantity of THC or CBD that is displayed on the label exceeds 5 mg, the product would be subject to a 15% variability limit (i.e. the container and any discrete units, if applicable, could not contain less than 85% of that amount, or more than 115% of that amount). If the quantity of THC or CBD that is displayed on the label is more than 2 mg but less than 5 mg, the variability limit would be 20%, and if the quantity of THC or CBD is less than 2 mg, the variability limit would be 25%.
- All cannabis extracts and cannabis topicals would be subject to a variability limit of 15%.
In addition, it is proposed that new rules would be established for edible cannabis, cannabis extracts, cannabis topicals, and cannabis accessories as described below.
A. Edible cannabis
All edible cannabis products would need to be shelf-stable (i.e. they could not require refrigeration or freezing).
Aside from cannabis itself, only food and food additives could be used as ingredients in edible cannabis, and the use of food additives would need to be in accordance with the limits and purposes that are prescribed for foods in the Food and Drug Regulations (FDR). Edible cannabis could not contain poisonous or harmful substances, nor could it be fortified with vitamins or mineral nutrients. Finally, if the edible cannabis product contains anything that would be considered unsafe and would cause the sale of a food regulated under the Food and Drugs Act (FDA) to be unsaleable, then its sale would similarly be prohibited under the Cannabis Act. It would similarly be prohibited to use any food described in a Temporary Marketing Authorization Letter issued under the FDR as an ingredient in edible cannabis.
The use of meat products, poultry products and fish as ingredients would be prohibited. Because dried products pose a lower risk from a food safety perspective than raw products, an exception to this prohibition would be provided for dried meats, poultry or fish, provided they are obtained from a person who is authorized to produce such products under provincial or territorial laws or the SFCA, and that they have a water activity of 0.85 or less at the time they are obtained. Furthermore, because of the increased risk of botulism associated with low-acid canned foods, it is proposed that the Regulations would prohibit the sale of edible cannabis in a hermetically sealed container if any constituent of the edible cannabis has a pH above 4.6 and a water activity higher than 0.85.
The use of ingredients containing naturally occurring caffeine would be permitted in edible cannabis provided the total amount of caffeine in a package does not exceed 30 mg. The proposed amendment would allow for the use of ingredients that contain naturally occurring caffeine, such as chocolate, tea, or coffee. The use of caffeine as a food additive would, however, be prohibited.
Furthermore, the Regulations would allow for a small concentration of ethyl alcohol in edible cannabis (that does not exceed 0.5% w/w), given that ethyl alcohol is often present as a by-product in fermented ingredients or products (e.g. vinegars).
B. Cannabis extracts
It is proposed that cannabis extracts could contain flavouring agents in addition to one or more carrier substances and any substance necessary to maintain the quality or stability of the cannabis product. Cannabis extracts could not contain ingredients that are sugars, sweeteners or sweetening agents. Furthermore, they could not contain any ingredient listed in Column 1 of Schedule 2 to the Tobacco and Vaping Products Act (which is a list of ingredients that are prohibited in vaping products). Any ingredient, other than a flavouring agent, used in the preparation of a cannabis extract that is intended to be inhaled would need to comply with a standard set out in one of the publications referred to in Schedule B to the FDA (which is a list of official publications that set out standards, such as the European Pharmacopoeia).
The use of ethyl alcohol would be permitted in cannabis extracts that are intended to be ingested (such as tinctures). However, it is proposed that the Regulations would prescribe a maximum package size of 7.5 g for all cannabis extracts that contain ethyl alcohol, and that this limit would apply regardless of the THC content of the product. As will be described under the "Packaging and labelling" section below, other controls would also apply to these products to address the risks associated with the co-use of alcohol and cannabis, as well as the risks associated with accidental consumption and overconsumption.
It is proposed that cannabis extracts could not contain any ingredients that may cause injury to the health of the consumer when the product is used as intended.
C. Cannabis topicals
It is proposed that cannabis topicals could not contain any ingredients that may cause injury to the health of the consumer when the product is used as intended. Health Canada's Cosmetic Ingredient Hotlist, which is a list of substances that are prohibited or restricted in cosmetics, could be used as a resource by licensed processors when looking to determine whether a particular ingredient could pose a risk of injury to the health of the consumer.
D. Cannabis accessories
Targeted amendments are proposed to ensure that cannabis accessories do not increase the potential for harm associated with cannabis products, and to establish dispensing limits for accessories containing certain cannabis extracts. More specifically, it is proposed that
- A cannabis accessory must not, through chemical means other than heating or combustion, alter or enhance the effects of the product, increase the potential for physical dependence on the product, or increase the toxicity of the cannabis product when used as intended.footnote 5
- The maximum amount of THC that could be dispensed per activation of a cannabis accessory containing an extract that is intended to be ingested, or for nasal, rectal, or vaginal use (e.g. a spray bottle), would be 10 mg. This provision would operate in concert with the proposed integrated dispensing mechanism, which is described in the "Proposed amendments to packaging requirements" section below.
Packaging and labelling
Part 7 of the Regulations sets out requirements that apply to cannabis products packaged and labelled for sale at the retail level. These packaging and labelling requirements aim to protect the health of young persons by restricting their access to cannabis and to protect young persons and others from inducements to use cannabis. The requirements also help to promote informed consumer choice and encourage the safe handling and storage of cannabis.
It is proposed that the Regulations would maintain the core plain packaging and labelling requirements that currently apply to all cannabis products, such as the standardized cannabis symbol, health warning messages, THC and CBD content, and child-resistant packaging. Specific additions and adjustments would be made to the Regulations to account for the new classes of cannabis and to address the public health risks associated with these new classes, in particular the risk of accidental consumption and overconsumption.
Mandatory information
As is currently the case, the Regulations would set out the mandatory information that must appear on the label of all cannabis products according to product class. The following label requirements would apply to the new classes of cannabis and to cannabis accessories containing those classes of cannabis.
A. Edible cannabis
Consistent with requirements that apply to food under the FDR, it is proposed that the following would be required on the label of edible cannabis products, in addition to the current labelling requirements, which apply to all cannabis products:
- A list of ingredients;
- The common name of the cannabis product;
- An indication of the source of an allergen or gluten, or that sulphites have been added to the product (alternatively, this information could appear as part of the ingredient list);
- A "durable life date" (more commonly known as a "best-before date"), which would apply only to edible cannabis products whose qualities are expected to deteriorate over a period of 90 days or less; and
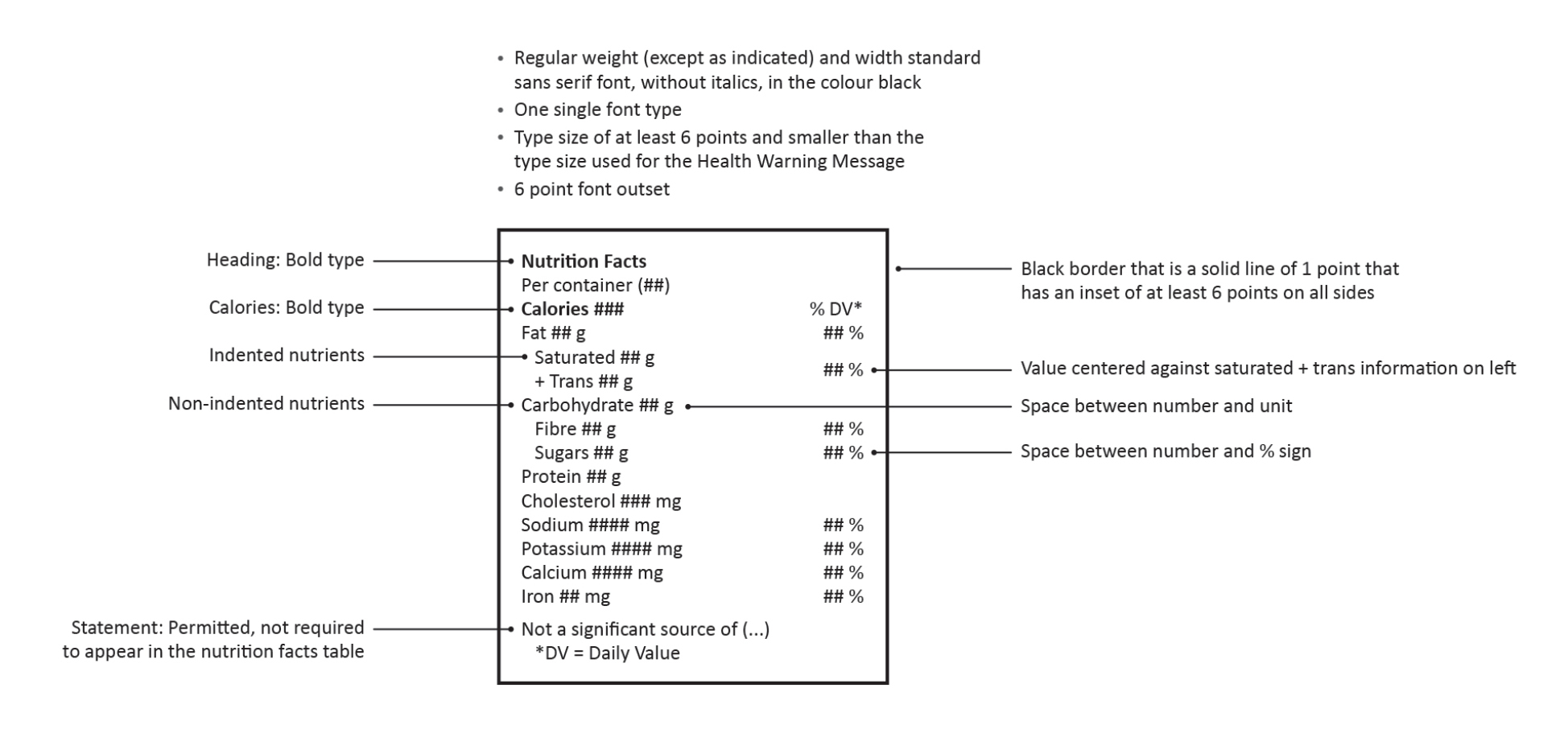
- A cannabis-specific nutrition facts table (NFT).
It is proposed that the cannabis-specific NFT would be modelled on the standard format NFT for pre-packaged food,footnote 6 incorporating the display of the energy value of the product (i.e. calories) as well as the amounts of the 12 core nutrients and, in some cases, the percent daily value (% DV), on a "per container" basis. The font size, font type, leading and spacing of the proposed cannabis-specific NFT would be consistent with other labelling requirements for cannabis products. The proposed requirement would allow consumers to make informed choices based on this information. For further information on the proposed NFT, please see Figure 1 below.
Figure 1. Proposed cannabis-specific nutrition facts table
B. Cannabis extracts
In addition to the current labelling requirements that apply to all cannabis products, it is proposed that a list of ingredients, the identity of the cannabis product in terms of its common name or function, and a list of allergens would be required on the label of cannabis extracts, as well as the intended use of the product (e.g. "for vaping").
C. Cannabis topicals
In addition to the current labelling requirements that apply to all cannabis products, it is proposed that a list of ingredients as well as the intended use of the product (e.g. "apply to skin") would need to appear on the label of all cannabis topicals. Directions for use would also need to appear on the label, but the content would not be prescribed by the Regulations. Furthermore, the following warning statement would need to appear on the label of all cannabis topicals: "Do not swallow or apply internally or to broken, irritated, or itching skin." footnote 7
D. Standardized cannabis symbol on vaping products and wrappers
The Regulations require that the standardized cannabis symbol appear on the label of cannabis products that contain more than 10 parts per million (ppm) THC (equivalent to 10 mcg per gram). It is proposed that the Regulations would be amended to also require the display of the standardized cannabis symbol on any cannabis accessory that contains a cannabis extract that is intended to be inhaled and that contains more than 10 ppm THC. For example, this would require vaping devices or vaping cartridges that contain a cannabis extract with THC to have the symbol directly on the device or cartridge. It is also proposed that the standardized cannabis symbol would need to be clearly and prominently displayed on the exterior surface of any wrapper that is in direct contact with edible cannabis, a cannabis extract, or cannabis topical that contains more than 10 ppm THC.
Reducing inducements to use cannabis (prohibitions on representations)
Consistent with the objective, as set out in section 7 of the Cannabis Act, to "protect young persons and others from inducements to use cannabis," it is proposed that the amended Regulations would prohibit the following representations on all product packages and labels:
- Representations regarding health benefits, including those that are currently permitted on food, such as "a healthy diet low in saturated and trans fat may reduce the risk of heart disease," or "oat fibre helps lower cholesterol" (all classes of cannabis);
- Nutrient content representations that go beyond those permitted in the list of ingredients and cannabis-specific NFT, including those that are currently permitted on food, such as "high source of fibre" or "low fat," or additional information pertaining to the vitamin or mineral content of the product (edible cannabis only); and
- Representations regarding cosmetic benefits, such as "reduces the appearance of wrinkles" or "softens skin" (all classes of cannabis).
It is proposed that the amended Regulations would also prohibit all representations that associate a cannabis product, its packaging or its labelling (including its brand element) with an alcoholic beverage. For example, it would be prohibited to use terms related to alcoholic beverages, such as "beer" or "wine," on cannabis products. It would similarly be prohibited for the name or logo of a company that manufactures alcoholic beverages to be used on a cannabis product. In addition to reducing inducements to use cannabis, this prohibition is felt to be necessary given the known health risks associated with the concurrent use of alcohol and cannabis.
Consistent with rules that apply to vaping products under the Tobacco and Vaping Products Act, it is proposed that the Regulations would prohibit the representation of certain flavours that are appealing to youth, such as dessert or confectionery flavours, on the packaging and labelling of cannabis extracts.
In addition, it is proposed that it would be prohibited to represent edible cannabis as being a suitable means of meeting the particular dietary requirements of an individual. For example, it would be prohibited to say that edible cannabis is suitable for people with diabetes, or as part of a low-calorie diet.
The prohibitions described above would be in addition to prohibitions set out in the Cannabis Act that prohibit the promotion, packaging and labelling of cannabis that could be considered reasonably appealing to young persons, the sale of cannabis products or cannabis accessories that have an appearance, shape or other attribute or function that could be appealing to young persons, as well as the use of false or misleading claims.
Proposed amendments to packaging requirements
It is proposed that the current plain packaging requirements for all cannabis products would be maintained, including the requirement for child-resistant packaging, with two minor adjustments.
First, it is proposed that an exception to the current prohibition on the use of a naturally occurring metallic colour on the external surface of an immediate container that is made of metal would be provided, which would allow for the use of containers such as metal beverage cans. Second, it is proposed that the exterior surface of any container in which a cannabis product is packaged would no longer need to have a matte finish, given that this requirement is incompatible with both the use of metal containers and the proposal to allow for the use of peel-back labels, footnote 8 which is described further below. Both proposed changes would facilitate compliance and provide additional flexibility for regulated parties.
In addition, the following specific additions and adjustments are proposed to account for the new classes of cannabis:
- The immediate container of cannabis extracts would need to be designed in such a way that the extract could not easily be poured, or drunk directly from the container (thereby mitigating against the risk of accidental consumption). For extracts in liquid form that are not intended to be inhaled and that contain at least 10 mg of THC, the immediate container would need to contain an integrated dispensing mechanism (e.g. a metered spray) that dispenses no more than 10 mg of THC, unless the extract is in the form of discrete units (such as a capsule).
- There would be a new requirement to use "food-grade" packaging (i.e. packaging that meets requirements set out in the FDR and the SFCR for food) for the immediate container of edible cannabis and for any wrappers.
- The co-packaging of edible cannabis and a food would be prohibited, as would the co-packaging of more than one class of cannabis in the same exterior container.
- An exterior container could not contain more than one immediate container, thereby preventing the sale of sampler packs.
- The immediate container of a cannabis product could not be pressurized; however, an exception would be provided for edible cannabis in liquid form, such as carbonated beverages.
As described under the “THC limits” section above, a number of restrictions on the amount of THC that could be in a package are proposed (e.g. 10 mg of THC per package of edible cannabis, 1 000 mg of THC per package for cannabis extracts and topicals). In addition, it is proposed that the following new maximum package sizes would apply:
- 7.5 g for all cannabis extracts that contain ethyl alcohol and are intended to be ingested (irrespective of the concentration of THC in that product); and
- 90 mL for all liquid cannabis extracts.
This is in addition to the maximum package size of 7.5 g that would apply to all "cannabis concentrates," by virtue of paragraph 108(f) of the current Regulations.
A. Allowances for smaller containers
Currently, the Regulations set out prescriptive display rules and plain packaging requirements that do not allow for the use of expanded panels on labels (such as peel-back and accordion panels), tags, or package inserts. To accommodate smaller containers, and based in part on feedback from regulated parties, the provinces and territories, and consumers, it is proposed that the Regulations would be amended to enable the use of expanded panels and alternative display formats for certain required information when the immediate container is too small to otherwise accommodate all required information on the exterior display surface. Tags and package inserts would continue to not be permitted.
The cannabis health warning messages, standardized cannabis symbol and information pertaining to the THC and CBD content of the product would always be required on the exterior display surface, regardless of the size of the container. However, information such as the packaging date, recommended storage conditions, the list of ingredients, and the NFT could be displayed on a peel-back or accordion panel. In such cases, regulated parties would be required to maximize the display of required information on the exterior display surface, and would be prohibited from adding any additional "voluntary" information to the alternative display. Brand elements appearing on the outer display could not be any larger than the minimum size of the standardized cannabis symbol (i.e. 1.27 cm by 1.27 cm), and no brand elements would be allowed on the peel-back or accordion panel.
These amendments are considered to be necessary given the proposed restrictions on the package sizes (e.g. the maximum package size of 7.5 g for cannabis extracts containing ethyl alcohol), and also in light of the new labelling requirements that increase the amount of required information (e.g. the NFT that is proposed for edible cannabis or the directions for use that are proposed for cannabis topicals).
Drugs containing cannabis
Part 8 and Part 9 of the Regulations set out rules for prescription drugs containing cannabis and medical devices containing cannabis or that are intended to be used with cannabis. No major changes are proposed to these parts of the Regulations.
Minor amendments to Part 8 are proposed that would remove the requirement to hold a drug establishment licence (DEL) under the FDR in order to be eligible to apply for a cannabis drug licence. This change is proposed because a small number of limited activities with drugs do not require a DEL (e.g. the sale and importation of a drug for use in clinical trials in humans). The grounds for refusal of a cannabis drug licence would be amended to include the applicant not holding a DEL at the proposed site, if one is required under the FDR, and the suspension or cancellation of an applicant's DEL, if one is required under the FDR, at the proposed site. Similarly, the grounds for suspension or revocation of a cannabis drug licence would be amended to clarify that the suspension or revocation of a DEL would only be grounds for suspension or revocation in cases where a DEL is required under the FDR.
Importation and exportation of cannabis
Part 10 of the Regulations deals with the importation and exportation of cannabis. As set out in the Cannabis Act, the import and export of cannabis is permitted only for medical or scientific purposes, or in respect of industrial hemp. No major changes are proposed to this part of the Regulations. Each importation and exportation of cannabis will continue to require a permit issued by the Minister. The following minor change is proposed: whereas import and export permits must currently set out "the description of the cannabis," they would now set out "a description of the cannabis."
Record keeping
Record-keeping requirements are set out in Part 11 of the Regulations.
Current record-keeping requirements that apply to cannabis oil (as set out in section 225) would be amended to apply to edible cannabis, cannabis extracts, and cannabis topicals.
It is also proposed to amend subsection 231(1) of the Regulations to specify that for each lot or batch of cannabis sold or exported, a document must be retained demonstrating that the cannabis meets the requirements set out in both Part 5 and Part 6 of the Regulations (as opposed to just Part 5, as is currently the case).
In addition to current record-keeping requirements, the following new requirements are proposed:
- Records would need to be kept pertaining to ingredients used in the production of edible cannabis, cannabis extracts, and cannabis topicals. footnote 9 In particular, it is proposed that the following records pertaining to ingredients would need to be kept by licensed processors:
- the name and business address of the person who supplied the ingredient;
- the date the ingredient was obtained or produced;
- a description of the ingredient, including its name (or, if applicable, its chemical name, common name, International Nomenclature Cosmetic Ingredient [INCI] name, or Chemical Abstracts Service [CAS] registry number); and
- a lot code or other unique identifier, if applicable.
- For cannabis extracts, it is proposed that a record be kept regarding the purpose of each ingredient (e.g. carrier substance, flavouring agent), as well as a description of the flavour of the product, if applicable.
- A record would need to be kept regarding every investigation undertaken by the QAP, including any proactive investigation of a possible risk of injury to human health or in response to a complaint received in respect of the quality of the cannabis. This record would also need to indicate any measures taken in response.
- Licence holders (all classes of cannabis, except plants and seeds) would be required to keep a record of any information that is obtained through testing that relates to the phytocannabinoid and terpene content of the cannabis product. Currently, licence holders are only required to keep a record of the quantity or concentration of THC, THCA, CBD, and CBDA. However, testing methodologies typically generate a more complete phytocannabinoid and terpene profile. Where this additional information exists, licence holders would be required to keep a record of it.
All of the above records would need to be kept for a period of two years, consistent with the retention periods for most existing record-keeping requirements (adverse reaction reports must be kept for 25 years).
Reporting
Reporting requirements are set out in both Part 12 of the Regulations and the Cannabis Tracking System Order.
No changes to Part 12 of the Regulations are proposed. Health Canada intends to update the ministerial order for the Cannabis Tracking System to reflect the new classes of cannabis.
Test kits
No changes are proposed to Part 13 of the Regulations, which deals with test kits.
Access to cannabis for medical purposes
No changes are proposed to Part 14 of the Regulations, which enables access to cannabis for medical purposes. Patients who have the authorization of their health-care practitioner and who are registered with the Minister or a federally licensed seller would benefit from the comprehensive range of new cannabis products that would be enabled through the current proposal, including several alternatives to smoking cannabis.
Transitional provisions
Part 15 of the Regulations deals with transitional provisions.
It is proposed that a six-month transition period would be provided for activities in relation to cannabis oil. During this proposed six-month transition period, cannabis oil could continue to be sold as a class of cannabis (by federal licence holders and provincially and territorially authorized distributors and retailers), subject to the current rules as they apply to cannabis oil. For example, cannabis oil would continue to be subject to the limit of 10 milligrams of THC per discrete unit, but the new limit of 1 000 milligrams of THC per package would not apply to cannabis oil during this transition period. Six months following the coming into force of the amended Regulations, cannabis oil would be removed as a class of cannabis under Schedule 4 to the Act.
It is also proposed that a six-month transition period for dried or fresh cannabis would be provided in relation to microbial and chemical contaminants (i.e. for a six-month period, microbial and chemical contaminants could remain within established limits for herbal medicines, rather than within limits appropriate for the intended use of the product, as described under the "Testing" section above). footnote 10
Amendments to Schedule 3
Schedule 3 to the Act (Equivalent Amounts) is used to determine, for example, how many grams of fresh cannabis would be equivalent to 30 grams of dried cannabis, for the purposes of determining the maximum amount of cannabis that an adult could legally possess in a public place.
Currently, both "cannabis solid concentrates" and "cannabis non-solid concentrates" are listed in Schedule 3. In both cases, the quantity that is deemed equivalent to 1 gram of dried cannabis is the same (i.e. 0.25 grams). Therefore, an amendment to Schedule 3 is proposed that would replace these two categories with a single category known as "cannabis concentrates." The term cannabis concentrate would be defined in the Regulations as "a substance that has a maximum yield percentage of greater than 3% w/w of THC, taking into account the potential to convert THCA into THC." This is consistent with the current definitions of both "cannabis solid concentrates" and "cannabis non-solid concentrates" in the Regulations.
Regulatory development
Consultation
Task Force on Cannabis Legalization and Regulation
As noted above, the Task Force was formed in June 2016 with a mandate to consult broadly with Canadians and to provide advice on the design of a new legislative and regulatory framework for cannabis in Canada. In its final report, A Framework for the Legalization and Regulation of Cannabis in Canada, the Task Force made 85 recommendations, including a number of recommendations specific to regulating edible cannabis and cannabis extracts, including extracts containing a higher concentration of THC.
Specifically with respect to edible cannabis, the Task Force recommended that
- Any product deemed to be appealing to children, including products that resemble or mimic familiar food items, or that are packaged to look like candy, should be prohibited;
- Packaging be implemented with standardized single servings, as well as a standardized cannabis symbol;
- A maximum amount of THC per serving be established, both on a per-serving and a per-product basis;
- Mixed products be prohibited; for example, cannabis-infused alcoholic beverages, or cannabis products with tobacco, nicotine or caffeine; and
- Labelling requirements that apply to food and beverage products should also apply to edible cannabis.
With respect to cannabis extracts containing higher concentrations of THC, the Task Force recommended that
- Regulatory oversight be provided in order to minimize the risks associated with the illegal production of such products (for example the use of highly combustible solvents, such as butane, and potentially toxic solvents, such as naphtha, which can be used to extract THC);
- Strategies be developed to encourage consumption of less potent cannabis;
- All cannabis products be required to include labels identifying levels of THC and CBD;
- A flexible legislative and regulatory framework be enabled at the federal level, which could adapt to new evidence and establish rules for limits on THC or other components; and
- The Government of Canada develop and implement factual public education strategies to inform Canadians about the risks of problematic use and to provide guidance on lower-risk use.
Parliamentary study of Bill C-45
During Parliamentary consideration and debate on Bill C-45, the Cannabis Act, the question of whether these new classes of cannabis should be permitted under the legal framework was the subject of considerable debate. Among those who supported their inclusion in the legal framework, there was debate as to whether these products should be legally available immediately upon the coming into force of the Act, or whether their legal sale should be enabled within a certain time frame. Ultimately, the House of Commons amended the Act to authorize the legal sale of "edibles containing cannabis" and "cannabis concentrates" no later than 12 months following the coming into force of the Cannabis Act.
During the debate in the Senate, some Senators expressed concern regarding the inclusion of cannabis products with a higher concentration of THC in the legal framework, given the greater health risks associated with such products. While there was some discussion of imposing a limit on THC, no such amendments were made to the legislation.
Consultations supporting the development of the Regulations
On November 21, 2017, Health Canada launched a 60-day public consultation to solicit public input and views on a proposed approach to developing regulations to support the coming into force of the Cannabis Act. To support the consultation, Health Canada published a detailed consultation paper entitled Proposed Approach to the Regulation of Cannabis. The consultation paper outlined a comprehensive series of regulatory proposals to help achieve the Government's public health and public safety goals of restricting youth access to cannabis, minimizing the harms of cannabis use, and preventing criminals and organized crime from profiting from the illegal production, distribution and sale of cannabis.
During the 60-day public comment period, Health Canada received more than 3 200 responses to an online questionnaire and 450 written submissions. Targeted consultations were also undertaken with interested parties, such as the provinces and territories, Indigenous governments and representative organizations, the cannabis industry (including both existing and prospective licensees), public health organizations, and patients and patient advocates. In total, 192 interested parties participated in the in-person round tables, and 343 interested parties participated in webinars. In addition, Health Canada held both multilateral and bilateral meetings with representatives of all provinces and territories, as well as a series of focused meetings with First Nations, Inuit, and Métis, to seek their feedback on the proposed regulatory approach.
On March 19, 2018, Health Canada published a report entitled Summary of Comments Received During the Public Consultation. In addition to highlighting areas where changes were being considered to the regulatory proposals, this document also provided advance notice of certain regulatory requirements — in particular those pertaining to the plain packaging and labelling of cannabis products.
A majority of those who responded to the November 2017 consultation paper indicated that the industry must be able to offer the same diversity of products that are available in the illegal market in order to be able to successfully displace the illegal market, and suggested that a broader range of products would provide alternatives to smoking cannabis. Many respondents felt that vaping products in particular should have been permitted through the first phase of the Cannabis Regulations.
In addition, respondents indicated that additional regulatory provisions would be required to address the unique public health and public safety risks associated with edible cannabis and extracts with a higher concentration of THC, such as provisions on quality control, THC limits, portion sizes, specific packaging and labelling requirements, and the use of highly combustible and/or potentially toxic solvents that are often used in the illegal manufacture of concentrated cannabis extracts. A small number of respondents felt that the regulations should be more restrictive in the types of cannabis products available through the legal system.
Among the proposals set out in the November 2017 consultation paper, it was proposed that a limit of 10 milligrams of THC per dose or unit for any cannabis product intended for ingestion be established, which most respondents supported. A maximum THC concentration for cannabis oil of 30 milligrams of THC per millilitre of oil was also proposed, and there were mixed views. Many respondents did not support the 30 milligrams per millilitre limit, stating that the limit was either too low or that there should not be a limit at all. Other respondents felt that the proposed limit was a prudent safeguard to mitigate risks of accidental overconsumption of a product class primarily intended for ingestion.
Modern treaty obligations and Indigenous engagement and consultations
Broadly speaking, Indigenous regulatory authority derives from different sources, including rights recognized and affirmed in section 35 of the Constitution Act, 1982, historic and modern treaties and land claim agreements, self-government agreements, and federal legislation, such as the Indian Act.
For example, section 81 of the Indian Act allows band councils to make by-laws in relation to the health of residents on the reserve and for the observance of law and order. First Nations would determine the types of restrictions they can establish, based on their interpretation of the authorities provided in the Indian Act. Such by-laws could co-exist with the Cannabis Act as long as they do not conflict with the Act or frustrate its purpose. The Government of Canada does not review, approve or maintain a list of by-laws enacted by First Nations under section 81 of the Indian Act.
Some First Nations and Inuit communities have negotiated self-government agreements and land claim agreements with the Government of Canada and provincial and territorial governments, which also contain legislative authority. Although self-government agreements vary across the country, the powers related to the making of by-laws they contain are generally similar to those found in the Indian Act. They allow for the making of by-laws in relation to protecting the health of the community and law and order, as well as in relation to the control or prohibition of "intoxicants." Land claim agreements usually set out limited authorities in relation to the management of settlement lands and resources covered under the agreements. In the context of the Cannabis Act and its regulations, Indigenous communities with that authority may therefore choose to review their agreements in order to determine what laws or regulations they may wish to pass within their communities.
Support for the self-determination of Indigenous peoples is a key objective of the Government of Canada. This must be balanced with the need to ensure that the legal and regulatory framework for cannabis, including criminal prohibitions, is applied consistently across the country. Therefore, similar to the Criminal Code, the Cannabis Act is a federal law of general application that applies to all people in Canada, including Indigenous peoples, whether they live on a reserve or not.
Indigenous engagement and consultations
The Government of Canada is committed to a renewed, nation-to-nation relationship with Indigenous peoples, based on recognition of rights, respect, co-operation and partnership. Therefore, Health Canada continues to engage and work closely with Indigenous governments, organizations and communities across the country to help ensure the specific interests of Indigenous peoples are carefully considered. Health Canada welcomes the views of Indigenous peoples with respect to the new classes of cannabis and the current regulatory proposal.
Engagement with Indigenous peoples has been ongoing throughout the development of the Cannabis Act and its regulations, beginning with the work of the Task Force in June 2016. Health Canada officials sought to broaden the reach of consultation efforts in the context of the November 2017 regulatory consultation paper entitled Proposed Approach to the Regulation of Cannabis through meetings with national and regional Indigenous organizations. The Minister also engaged with the Assembly of First Nations, Inuit Tapiriit Kanatami, and Métis National Council to seek their active participation in public consultations on the proposed regulatory approach. The Government has also engaged extensively with Indigenous leadership, organizations and communities to provide information on the legislation and regulations and discuss the unique interests of First Nations, Inuit and Métis. As of November 1, 2018, Government of Canada officials have participated in approximately 85 engagement sessions with Indigenous leaders, organizations and communities. Through these discussions, First Nations, Inuit and Métis organizations and leaders have expressed a wide range of diverse views and objectives regarding cannabis legalization and regulation. Four themes have arisen consistently:
- public health and public education;
- taxation and revenue generation;
- Indigenous authorities over activities related to cannabis; and
- economic development.
The Government understands that there is significant interest among Indigenous communities in Canada regarding the Cannabis Act and its regulations and has taken important steps to address specific interests expressed by Indigenous communities and organizations. The Government will continue to work closely with Indigenous communities and organizations to ensure that their specific needs and interests are carefully considered throughout the implementation of the Cannabis Act.
To support the consultation on the draft Regulations described herein, Health Canada intends to publish a notice in the First Nations Gazette, with the goal of ensuring a robust consultation of Indigenous peoples. In addition, Health Canada will directly notify national Indigenous organizations, political-territorial organizations, modern treaty holders and self-governing Nations of the consultation and invite their input. All input received through the public consultation will inform the development of the final Regulations.
Instrument choice
Incorporation by reference
A number of documents have been incorporated by reference (IBR) as part of the Regulations. Of the existing IBR documents, the intent is to update three of them to coincide with the publication of the final Regulations in the Canada Gazette, Part II.
One of those documents is the Consumer Information – Cannabis document. This document, which was developed by Health Canada, supports one of the explicit purposes of the Cannabis Act, which is to enhance public awareness of the health risks associated with cannabis. It represents one of several tools being used to help educate consumers on cannabis and its effects and risks, and to promote informed and responsible use. This document is intended to be provided to all consumers when they purchase cannabis products. Health Canada plans to update the Consumer Information – Cannabis document to include specific messages pertaining to the health risks of edible cannabis, as well as cannabis extracts with a higher concentration of THC.
In a similar vein, Health Canada plans to update the Cannabis health warning messages to include new health warning messages pertaining to edible cannabis. Health Canada is currently considering options, including new health warning messages, to more effectively distinguish between lower THC-concentration and higher THC-concentration cannabis products, and thereby to promote informed consumer choices.
In addition, Health Canada intends to update the IBR document entitled Tolerance Limits for the Net Weight and Volume Declared on Cannabis Product Labelling to account for larger package sizes (currently, this document sets out a tolerance limit of 10% for package sizes of 2 grams or millilitres or less, and 5% for package sizes of more than 2 grams or millilitres).
In addition to the three updates to existing IBR documents, it is proposed that a new document entitled Directory of Nutrition Facts Table Format for Edible Cannabis would be incorporated by reference as part of the amended Regulations. This IBR document would set out specific requirements pertaining to the NFT, as described in the "Description" section above.
Finally, it is proposed that the current IBR document entitled Limits for Residual Solvents in Cannabis Products would no longer be incorporated by reference as part of the Regulations. Instead, given the proposal to treat solvent residues in the same way as other chemical contaminants, the limits for residual solvents and other chemical contaminants would be found in any of the publications listed in Schedule B to the FDA (which is a list of official publications that set out standards, such as the European Pharmacopoeia).
Baseline scenario
As explained previously, as per section 193.1 and subsection 226(2) of the Act, "edibles containing cannabis" and "cannabis concentrates" will automatically be added to Schedule 4 to the Act on October 17, 2019, unless the Governor in Council authorizes their addition to Schedule 4 in advance of this date, by Order.
For the purposes of this RIAS and the accompanying cost-benefit analysis (CBA), the baseline scenario assumes that section 193.1 of the Act would come into force automatically on October 17, 2019, and that the Government would adopt an approach that would effectively rely on other frameworks to regulate these new classes of cannabis.
Even under the baseline scenario, minor regulatory amendments would be required. This is because certain provisions of the current Regulations, such as restrictions on mixing cannabis with other ingredients, would effectively preclude the production of the new classes of cannabis, regardless of the fact that their legal sale would be permitted. For the purposes of the baseline scenario, it is assumed that amendments would be made to any provisions preventing the production of the new classes of cannabis. In particular, the baseline scenario assumes that the current maximum yield quantity of 30 milligrams of THC per millilitre of cannabis oil (equivalent to 3%) would be removed, thereby enabling the production and sale of cannabis oil products with a higher concentration of THC (i.e. "cannabis concentrates"), and that the use of ingredients in "edible cannabis" would be permitted. In the baseline scenario, such amendments would allow for the production of a limited suite of edible and concentrated products, but not the same comprehensive range of cannabis products that would be permitted under the proposed regulatory scenario. Under the baseline scenario, it is assumed, for example, that cannabis oil containing higher concentrations of THC would be permitted, but that other cannabis extracts that are not in liquid form at room temperature would not (e.g. wax, hash).
Because edible cannabis meets the definition of "food" under the FDA (and, by extension, the SFCA), the baseline scenario assumes that "edible cannabis" would be subject to the Safe Food for Canadians Regulations (SFCR) and the Cannabis Regulations. However, the FDA and its regulations would not apply. This is because the Cannabis Exemption (Food and Drugs Act) Regulations exempt cannabis produced by authorized persons from the application of the FDA. It is likely that this would result in confusing and potentially conflicting rules, which would increase the risks to public health and make compliance and enforcement difficult and potentially ineffective.
The baseline scenario also assumes that a specific regulatory scheme designed to address the public health and public safety risks posed by edible and concentrated forms of cannabis would not be created, which is clearly inconsistent with the objectives of the Act.
Regulatory scenario
In contrast, under the proposed regulatory scenario, three new classes of cannabis would be added to Schedule 4 to the Act (i.e. edible cannabis, cannabis extracts, and cannabis topicals). Amendments would be made to the Regulations to address the public health and public safety risks of these new classes of cannabis, resulting in benefits for Canadians. Other amendments to the Regulations would be made to permit a comprehensive range of cannabis products, consistent with the Government's objective of displacing the illegal market.
To reduce the risk of duplication and overlap between regimes and to create clarity and predictability for regulated parties, it is proposed that these new classes of cannabis would be regulated exclusively under the Cannabis Act and the Cannabis Regulations. More specifically, Health Canada intends to issue a notice of intent clarifying that edible cannabis would not be treated as a "food" and that it would be controlled exclusively under the Cannabis Act and its regulations (and, by extension, that edible cannabis will not be subject to the SFCA and its regulations). footnote 11
Under the regulatory scenario, it is proposed that relevant provisions of other frameworks, such as those for food, cosmetics, and vaping products under the SFCA, the FDA, and the Tobacco and Vaping Products Act, would be incorporated or adapted into the Cannabis Regulations, thereby creating consistency between frameworks.
Regulatory analysis
Benefits and costs
It is estimated that the proposed amendments to the Regulations would result in a net cost to Canadians of approximately $40.5 million net present value (PV), in 2017 dollars. These costs, which would be borne entirely by the regulated industry (in particular, licensed processors), are primarily associated with costs to comply with new regulatory requirements for packaging and labelling, good production practices, testing, and record keeping. Despite the net cost, the qualitative benefits attributed to displacing the illegal market and providing adult consumers and registered clients of licensed sellers of cannabis for medical purposes with access to quality-controlled edible cannabis, cannabis extracts, and cannabis topicals can be expected to outweigh the net cost to Canadians of the current regulatory proposal.
Market projections
The CBA conducted for the Cannabis Regulations (i.e. the market projections described in the RIAS footnote 12 published in the Canada Gazette, Part II, on July 11, 2018) considered total demand for cannabis products, and did not distinguish between classes of cannabis that became legal to sell in Canada by authorized persons on October 17, 2018, and the proposed new classes of cannabis. In other words, the market projections included in the July 11, 2018, RIAS accounted for edible cannabis, cannabis extracts, and cannabis topicals.
According to a recent report, footnote 13 an average of 43% of the total cannabis market in Colorado, California and Oregon between January and July of 2018 was made up of cannabis products other than dried cannabis (based on the top 10 products). Assuming the trend is consistent in Canada, the new classes of cannabis will likely represent over time a significant portion of the total market.
Analytical approach
The Cabinet Directive on Regulation requires departments to analyze the costs and benefits of proposed federal regulations. To measure these impacts, the benefits and costs are estimated by comparing the incremental change from the current regulatory framework (i.e. the "baseline scenario") to what is anticipated to occur under the proposed new regulatory approach (i.e. the "regulatory scenario").
The baseline scenario, which is described under the "Instrument choice" section above, assumes that edible cannabis would be subject to the Cannabis Regulations and the SFCR, but that the FDA and its regulations would not apply. In contrast, under the regulatory scenario, edible cannabis would not be subject to the SFCR. Therefore, for the purposes of this CBA, any of the proposed regulatory requirements that have been adapted from the SFCR in the regulatory scenario do not represent a cost to industry. However, any of the proposed requirements adapted from the Food and Drugs Regulations in the regulatory scenario do represent a cost to industry. The projected regulatory scenario represents the most likely outcome of the proposed amendments and additions, based on current information.
The period of analysis for this CBA covers the 10-year period from 2019–2020 to 2028–2029, with each year starting on October 17 and ending on October 16 of the following calendar year. As per Treasury Board of Canada Secretariat guidelines, footnote 14 the CBA only assesses incremental impacts directly related to a regulatory requirement. Any impact that is not associated with a regulatory requirement is considered out of scope for the purposes of the CBA. A 7% discount rate is used to estimate the present value of the incremental costs and incremental benefits. All values are expressed in 2017 constant dollars and reported for the 10-year period unless otherwise stated.
A summary of the CBA is provided herein. A copy of the full CBA report is available upon request from cannabis@canada.ca.
Assessing regulatory impacts
Regulatory impacts in this analysis have been estimated using two approaches: quantitative analysis, where possible, and qualitative assessments. For the quantitative analysis, Health Canada relied on responses to a questionnaire distributed to licence holders in February 2018. Where data was not available from the questionnaire, internal Health Canada data, as well as data from previous cost-benefit analyses (such as the CBA supporting the Safe Food for Canadians Regulations) and data from the United States were used as proxies to quantify the impacts of the proposed regulatory changes. When quantification of estimates was not possible, qualitative assessments have been provided.
Health Canada intends to send another questionnaire to federal licence holders early in 2019. The results of this second survey will help to round out and refine the CBA, and will hopefully allow for the quantification of some of the costs and benefits that have been discussed qualitatively below prior to the publication of final regulatory amendments in the Canada Gazette, Part II.
Affected stakeholders
As mentioned above, it is proposed that no special licence would be required in order to produce edible cannabis, cannabis extracts, and cannabis topicals. Instead, a cannabis processing licence (either standard or micro) would be required in order to manufacture these classes of cannabis and to package and label final products for sale to consumers. Licensed processors would be subject to all of the new rules applying to the production of the new classes of cannabis. Therefore, costs to the industry associated with the proposed amendments to the Regulations would be borne primarily by licensed processors. At the same time, these licensed processors also stand to benefit financially from the sale of the new classes of cannabis.
Current consumers of edible cannabis, cannabis extracts and cannabis topicals purchased from the illegal market, as well as current consumers of cannabis products purchased legally (including those who have the authorization of their health-care practitioner to access cannabis for medical purposes) would also be impacted by the current proposal. The impacts on consumers are discussed further as part of the analysis below.
Government costs are not described as part of this CBA, as total government costs were included in the CBA conducted for the Cannabis Regulations. As will be described further below, it is expected that the Government of Canada would benefit from a small cost savings in the regulatory versus the baseline scenario.
Key assumptions
The following assumptions were made when developing the CBA:
- As mentioned above, the CBA conducted for the Cannabis Regulations considered the total demand for cannabis products (including edibles, extracts, and topicals). As a result, this CBA assumes that there would be no new market entrants (i.e. no increase in the total number of federal licence holders) beyond those that were previously forecasted to enter the market.
- Based on projections from the previous RIAS and CBA, it is assumed that there would be 435 licensed processors by the final year of this analysis (2028–2029). This projected number is itself based on a number of assumptions, including that the rate at which new licences would be granted would be consistent with the rate at which licences were being granted under the former Access to Cannabis for Medical Purposes Regulations. These assumptions likely underestimate the number of licences that will be granted, based on improvements and resources that Health Canada has put in place to improve the review of licence applications, as well as potential efficiencies to be gained through the new Cannabis Tracking and Licensing System.
- For the purposes of this CBA, an assumption has been made that all licensed processors would manufacture all of the new product classes. This is not to say that there would not be room in the market for licensees who may choose to specialize in the processing of one or more of the new classes of cannabis (e.g. cannabis extracts only, or edible cannabis only). However, since no additional licence would be required for a licensed processor to manufacture all classes of cannabis, it is difficult to estimate how many would choose to expand their product lines and how many would not. This assumption may result in an overestimate of the costs to industry associated with the current regulatory proposal.
Assessment of costs and benefits
It is estimated that the proposed amendments to the Regulations would result in a total cost of $44.5 million and a total benefit estimated at $4.06 million. Taken together, the proposed amendments are estimated to generate a net cost of $40.5 million PV over 10 years (or $5.8 million annually). Total costs are attributed to federal licence holders only (primarily licensed processors), whereas the total benefits are attributed to both industry (including federal licence holders and provincially and territorially authorized distributors and retailers) and consumers, with the majority of benefits going to consumers.
The sale of edible cannabis, cannabis extracts and cannabis topicals (with the exception of cannabis oil) is currently illegal in Canada. However, a wide range of products are currently being sold in the illegal market. Should the legal sale of such products by authorized persons be permitted by the Governor in Council, it is expected that products produced by the legal industry would capture an increasing share of the market in the years following the coming into force of the amendments to Schedule 4 to the Act and to the Regulations. In other words, it is expected that the current proposal would help to achieve the Government's objective of displacing the illegal market.
For the purpose of this CBA, the displacement of the illegal market is considered a transfer of cannabis revenue from the illegal to the legal market. Although federal licence holders (as well as provincially and territorially authorized retailers) can be expected to benefit from this transfer, as they would accrue profits from the sale of these new cannabis products, the economy-wide impact is neutral; therefore, the benefit has not been quantified as part of this CBA.
While it can be expected that some displacement of the illegal market would occur even under the baseline scenario (given that the baseline scenario assumes that a minimal set of regulatory amendments would be made to allow for the production of edible cannabis and cannabis oil with a higher concentration of THC than is permitted under the current Regulations), the current regulatory proposal would authorize the production of a much broader suite of new cannabis products than would be allowed under the baseline scenario. Specifically, under the baseline scenario, only concentrated forms of cannabis oil would be permitted (not the broader category of cannabis extracts), and the vast majority of cannabis topicals would not be permitted.
It should be noted that there are a number of products currently available from the illegal market that would not be permitted under the current regulatory proposal. This would include products that pose a risk of injury to human health, products that could have serious effects if consumed accidentally, products that do not have restrictions in place to prevent overconsumption (e.g. no THC limits, no integrated dispensing mechanism), and products that are appealing to children. While these strict restrictions may be viewed as a "cost" to regulated parties, it is expected that any such costs would be outweighed by the significant benefits to public health and public safety. Furthermore, it is anticipated that alternatives to many of these riskier products would eventually become available through the legal market.
In order for federal licence holders to produce these new classes of cannabis and benefit financially from their sale, they would need to meet all of the proposed new regulatory requirements and incur the applicable compliance and administrative costs associated with the amended Regulations. These regulatory costs are described in further detail below.
Net costs footnote 15
A. Packaging and labelling requirements
Given that it is currently illegal to sell edible cannabis, cannabis extracts, and cannabis topicals in Canada (with the exception of cannabis oil), it is reasonable to assume that the packages and labels for most of these products have not yet been designed or produced.
In both the baseline and regulatory scenarios, a one-time cost would be incurred to design and produce packages and labels for the new classes of cannabis. This analysis contends that because the design and production costs would be incurred regardless of the regulatory requirements, no incremental costs to regulated parties are associated with the proposed labelling requirements described earlier. All costs related to the placement of new mandatory information on the package or label (including the list of ingredients, the list of allergens, the durable life date, the cannabis-specific NFT, and the intended use of the product) are not considered to be incremental in comparison with the baseline scenario.
This assumption is consistent with cost-benefit analyses conducted for other packaging and labelling regulations, which have also assumed that companies regularly redesign their packaging and labelling in order to remain competitive and relevant. In a CBA guide to the costs and benefits of nutrition labelling, the United Nations Food and Agriculture Organization (FAO) stated: "If the imposition of mandatory nutrition labelling provides for a transition period, during which time industry can exhaust their existing stock of labels, it is possible to minimise redesigning and reprinting costs. Industry can then include the required nutrition information on future labels as part of routine redesigning/updating of food labels, and thereby minimise the costs arising from complying with mandatory labelling." footnote 16
One aspect of the proposed packaging and labelling requirements that does impose an incremental cost on industry is the Nutrition Facts Table (NFT). While the placement of the NFT on the package is not considered an incremental cost, the development of the NFT is. footnote 17 Licensed processors would be required to conduct an analysis of the number of calories, fat, carbohydrates, protein, etc., in edible cannabis products, in order to accurately specify nutrient content in the cannabis-specific NFT.
This cost would be assumed by licensed processors on an ongoing basis over the 10-year period. The cost is a one-time cost per stock keeping unit (SKU), with new SKUs expected to enter the market on an annual basis. For licensed processors, it is anticipated that complying with this requirement would result in a net cost of $15.2 million PV over 10 years (or $2.2 million annually).
B. Good production practices
As mentioned above, for the purposes of this CBA, any of the regulatory requirements that have been adapted from the SFCR are not considered to represent a cost to industry, given the way in which the baseline and regulatory scenarios have been defined. In other words, given that most of the proposed new good production practices (GPP) requirements are adapted from the SFCR, the incremental costs to industry associated with the proposed new GPP requirements are minimal.
One requirement that was not adapted from the SFCR is the proposed amendment to section 80 of the Regulations, whereby standard operating procedures would apply equally to cannabis (as is currently the case) and to ingredients used in the production of cannabis products. For licensed processors, it is anticipated that complying with this proposed new requirement would result in a net cost of $20.6 million PV over 10 years (or $2.9 million annually).
C. Solvent testing
The current Regulations require that cannabis oil be tested for the residues of solvents used in its production. Although minor adjustments to this requirement have been proposed, edible cannabis, cannabis extracts, and cannabis topicals would nonetheless need to be tested for solvent residues. It is anticipated that licensed processors would assume a net cost of $7.9 million PV over 10 years (or $1.1 million annually) to conduct solvent testing for the new classes of cannabis.
D. Record keeping
It is proposed that edible cannabis, cannabis extracts and cannabis topicals would be subject to all of the same record-keeping requirements that currently apply to all cannabis products, as well as to some additional requirements as described under the "Description" section above. The additional time required for the record keeping of these new products (over and above current record-keeping requirements) is assumed to be minimal. The CBA conducted for the Cannabis Regulations assumed that the amount of time needed to comply with current record-keeping requirements would be 30 minutes per week. The current CBA assumes that the additional proposed record-keeping requirements described herein would add an additional 10 minutes to the weekly record-keeping time. Records would be kept electronically, following the same procedures being used for the record keeping of products under the current Regulations. It is anticipated that the incremental cost of the proposed new record-keeping requirements for licence holders will be $689,816 PV over 10 years (or $98,214 annually).
E. Understanding the Regulations
Should the proposed amendments come into force, certain aspects of the regulatory framework for activities related to cannabis would change. This is especially true in respect of edible cannabis, cannabis extracts and cannabis topicals. In order to comply with any new or amended regulatory requirements, licence holders would spend time reviewing and understanding the requirements that would affect their day-to-day business operations. This CBA assumes that it would take the same amount of time, per licence holder, to read and understand the amended Regulations as it did for them to read and understand the current Regulations. It is anticipated that the measures taken to understand the amended Regulations would cost $190,021 PV over 10 years (or $27,055 annually).
Qualitative costs
A. Integrated dispensing mechanism
As noted in the "Description" section above, it is proposed that the immediate container of a cannabis extract in liquid form that is not in discrete units and that is not intended to be inhaled would be required to contain an integrated dispensing mechanism. The proposed requirement is intended to protect adult consumers and youth alike from the risks associated with overconsumption and accidental consumption. It is anticipated that the requirement to build an integrated dispensing mechanism into immediate containers would impose an incremental cost on licensed processors; however, Health Canada does not have the data necessary to quantify this cost at this time.
B. Limit of 1 000 milligrams of THC per package for cannabis extracts and cannabis topicals
As noted in the "Description" section above, it is proposed that the maximum amount of THC that would be allowed in a package for cannabis extracts and topicals would be 1 000 mg. As no such limit exists currently for cannabis oil, it is anticipated that this requirement would impose an incremental cost on licensed processors; however, Health Canada does not have the data necessary to quantify this cost at this time. It is proposed that a six-month transition period would be provided for activities in relation to cannabis oil, including this requirement. The proposed measure is considered an important safeguard in mitigating against the risk of accidental consumption and overconsumption.
C. Standardized cannabis symbol on vaping products
As noted in the "Description" section above, it is proposed that the display of the standardized cannabis symbol would be required on any cannabis accessory that contains cannabis extract that is intended to be inhaled and that contains more than 10 ppm THC, such as a vaping device or a vaping cartridge. This proposed new requirement is expected to result in a cost to federal licence holders; however, Health Canada does not have the data necessary to quantify this cost at this time.
D. Prohibition on manufacturing edible cannabis in a food facility
As noted in the "Description" section above, it is proposed that the production of edible cannabis at a site where conventional food products are also being manufactured for sale could only be done provided the edible cannabis were being produced within another building within the licensed site. It is anticipated that this proposed requirement would impose an incremental cost on licensed processors; however, Health Canada does not have the data necessary to quantify this cost at this time.
E. Prohibitions on representations
As noted in the "Description" section above, a number of prohibitions on representations have been proposed, including health benefit claims, nutrient content claims, cosmetic benefit claims, representations that associate a cannabis product with an alcoholic beverage, the representation of certain flavours that are appealing to youth in cannabis extracts, and the representation of edible cannabis as being a suitable means of meeting dietary requirements. It is anticipated that these proposed prohibitions would impose an incremental cost on licence holders, as the prohibitions would restrict the ability of licence holders to market their products. However, the proposed prohibitions are entirely consistent with the objective, as set out in the Act, to "protect young persons and others from inducements to use cannabis."
Net benefits
A. No SFCR Licence
Under the baseline scenario, licensed processors would be subject to the Cannabis Act and the SFCA. Therefore, they would be required to obtain licences under the Cannabis Regulations and the SFCR. footnote 18 Under the regulatory scenario, licensed processors would not be required to obtain a licence under the SFCR.
This would result in a reduction in the administrative burden associated with the process of applying for a licence under the SFCR. It is anticipated that licensed processors would benefit in the amount of $49,755 PV over the 10-year period (or $7,084 annually) in administrative costs savings as a result.
Furthermore, licensed processors would not be required to pay the $250 fee to apply for a licence under the SFCR. It is anticipated that licensed processors would benefit in the amount of $401,088 PV over the 10-year period (or $57,106 annually) as a result of this fee savings.
Finally, the Government would benefit by saving the costs related to providing the service of administering and processing licences under the SFCR, which are not fully cost-recovered. In 2017, the Canadian Food Inspection Agency (CFIA), which will administer the SFCR when they come into force in January 2019, conducted consultations on a proposed restructuring of its cost recovery regime. During these consultations, it was noted that CFIA fees are currently well below the cost to deliver services. The CFIA is continuing to examine its cost recovery structure and will consult stakeholders again before any restructuring takes place. For the purposes of this CBA, it is assumed that the fees charged to the industry reflect 10% of the government costs of providing the service to the industry. Therefore, it is estimated that the proposal to not require licensed processors to also hold a licence under the SFCR would result in a savings to Government of $2,250 per licence, or a total benefit of $3.6 million PV over the 10-year period (or $513,953 annually).
Qualitative benefits
A. Public health
A number of regulatory provisions have been proposed to address the public health and public safety risks posed by the new classes of cannabis. The provisions are intended to protect public health by reducing the appeal of such products to youth, and the risks of overconsumption and accidental consumption. Furthermore, it is proposed that the amended Regulations would contain a number of provisions designed to reduce the risk of foodborne illness associated with edible cannabis, including a number of provisions adapted from the SFCR (e.g. good production practices) and the FDR (e.g. food-grade packaging).
There is currently no legal commercial source of supply for consumers who may wish to purchase edible cannabis, cannabis extracts, and cannabis topicals, in Canada (with the exception of cannabis oil). However, such products are readily available from the illegal market. Enabling the legal production and sale of these new classes of cannabis products would allow adult Canadians to have access to a legal, quality-controlled and strictly regulated supply of such products. This would represent a benefit for those who are currently purchasing such products from the illegal market.
Current consumers of legal cannabis products (including registered clients of licensed sellers of cannabis for medical purposes) would also be affected by the current proposal, as they would be able to choose from among a much broader suite of legal products. Some of these products would present a reduced health risk, such as alternatives to smoking cannabis. However, some of the new products that would be authorized by the current proposal represent a greater health risk, such as products with a higher concentration of THC. Public education efforts will be a critical component of mitigating the health risks of such products.
Consumers would also benefit from the proposal to prohibit representations that associate a cannabis product, its packaging or its labelling with an alcoholic beverage. This proposed restriction aims to decrease the potential normalization of alcohol and cannabis concurrent use, given that combining cannabis with alcohol increases impairment. Furthermore, recognizing the popularity and usage rates of alcohol and other related products (e.g. alcoholic caffeinated energy beverages) among Canadian adults and youth, this requirement aims to decrease opportunities for promotion or inducements to use cannabis.
B. Public safety
The proposed amendments to the Regulations would enable a broader suite of products than would be permitted under the baseline scenario. Therefore, it is anticipated that the proposed regulatory changes would prove more effective in terms of meeting the Government's objective of displacing the illegal market than would be expected under the baseline scenario. In addition, the requirement for licence holders to keep a detailed record of the new cannabis classes they sell, distribute, import, export, and keep in inventory would also act to prevent diversion of legal cannabis to the illegal market. The records would enable Health Canada inspectors to check actual inventories against what is recorded to verify that there are no anomalies.
In addition, a number of provisions under the Regulations, particularly those in Part 5 (pertaining to good production practices), help to ensure the proper use of solvents in the production of cannabis extracts. These provisions would minimize the risk of fires and explosions that are often associated with the illegal production of such products.
C. Allowances for smaller packages
As noted under the "Description" section above, it is proposed that the use of expanded panels (i.e. peel-back and accordion panels) for the display of certain mandatory information would be permitted. This amendment would apply equally to existing and new classes of cannabis, thereby allowing for smaller packages than under the current Regulations. This allowance for smaller packages is expected to result in environmental benefits, as described below in the "Strategic Environmental Assessment" section, as well as cost savings for licensed processors who are packaging and labelling cannabis products. However, Health Canada does not have the data necessary to quantify this benefit at this time.
Summary: Net benefit of proposed amendments to the Cannabis Regulations
In summary, the proposed amendments to the Regulations are estimated to generate a net cost to federal licence holders of $40.5 million PV over the 10-year period (or $5.8 million annually). In contrast, the potential public health and public safety benefits resulting from the current proposal are considerable, even if they cannot be quantified. It is expected that these benefits would outweigh the costs. The following table provides a detailed cost-benefit statement.
| Base Year 2019–2020 |
Year 4 2022–2023 |
Year 7 2025–2026 |
Year 10 2028–2029 |
Total (PV) | Annualized Average | |
|---|---|---|---|---|---|---|
| A. Quantified impacts (2017 constant dollars, CAN$) Benefits Benefits to Government |
||||||
| SFCR licence — Application processing savings | 623,939 | 197,818 | 786,598 | 197,818 | 3,609,790 | 513,953 |
| Benefits to federal licence holders | ||||||
| No SFCR licence — Fee and administrative savings | 77,927 | 24,706 | 98,242 | 24,706 | 450,843 | 64,190 |
| Total benefits of the amended Regulations | 701,865 | 222,525 | 884,840 | 222,525 | 4,060,633 | 578,143 |
| Costs Costs to federal licence holders |
||||||
| Packaging and labelling requirements | 5,387,664 | 1,500,733 | 1,480,064 | 1,478,476 | 15,184,347 | 2,161,909 |
| Good production practices | 13,503,725 | 33,009 | 0 | 0 | 20,575,896 | 2,929,545 |
| Solvent testing | 587,832 | 1,044,808 | 1,209,495 | 1,400,142 | 7,874,022 | 1,121,084 |
| Record keeping | 62,697 | 98,919 | 98,421 | 98,315 | 689,816 | 98,214 |
| Understanding the Regulations | 124,709 | 305 | 0 | 0 | 190,021 | 27,055 |
| Total costs of the amended Regulations | 19,666,625 | 2,677,773 | 2,787,980 | 2,976,933 | 44,514,103 | 6,337,807 |
| Net impact of the amended Regulations | −18,964,760 | −2,455,248 | −1,903,140 | −2,754,408 | −40,453,470 | −5,759,664 |
| Small business impact (cost) | 17,699,963 | 2,409,996 | 2,509,182 | 2,679,240 | 40,062,693 | 5,704,026 |
| B. Qualitative impacts | ||||||
Benefits Public health
|
||||||
Sensitivity analysis
A sensitivity analysis was conducted to show the estimated net impacts of proposed amendments to the Regulations at a 3%, 7% and 10% discount rate (7% was used in this analysis). At a 3% discount rate, the net impact would be a $43.8 million PV cost over 10 years, while a 10% discount rate would result in a $38.4 million PV cost over the 10-year period. As seen in Table 1, the 7% discount rate results in a net cost of $40.5 million PV over 10 years.
Small business lens
The cannabis industry is currently comprised mostly of small businesses. Based on internal Health Canada data and input from responses to questionnaires that were distributed to industry stakeholders in February 2018, it is assumed that 90% of licence holders will meet the definition of small business footnote 19 throughout the period from 2019–2020 to 2028–2029.
The Cannabis Regulations and the current regulatory proposal were both developed with small businesses in mind, which addresses the requirement to consider approaches that address small business needs. The requirements described herein are designed to address the public health and public safety risks associated with the new classes of cannabis. As such, it is considered important that all licence holders who opt to produce the new classes of cannabis comply with the proposed new requirements pertaining to those classes. However, the licensing framework provides alternative compliance approaches for smaller producers. First, it would be possible for holders of a micro-processing licence (as opposed to a standard processing licence) to produce, package and label the new classes of cannabis. Micro licence holders are subject to somewhat reduced physical security requirements as compared with standard licence holders, reflecting differences in the risk of diversion related to the scale of the operation, which reduces upfront capital costs and facilitates compliance. Second, given that a number of the proposed new requirements are specific to one or more of the new classes of cannabis, small businesses could opt to specialize in, for example, only the production of cannabis extracts, and would not need to comply with requirements pertaining to edible cannabis.
Of the $44.5 million PV cost to industry over the 10-year period, roughly $40 million PV (2017 base year) will be assumed by small businesses ($5.7 million annually). The cost per small business decreases from roughly $71,000 in Year 1 of the analysis, to just $6,900 in the final year. Based on the projected legal grams produced per year, this translates to $0.07 per gram in Year 1, and less than one cent per gram in Year 10.
| Number of small businesses impacted | 392 (final year) | |
|---|---|---|
| Number of years | 10 years (2019–2020 to 2028–2029) | |
| Base year for costing | 2017 | |
| Compliance costs | Annualized Value | Present Value |
|
$1,945,718 | $13,665,912 |
|
$2,636,591 | $18,518,306 |
|
$1,008,976 | $7,086,620 |
|
$21,915 | $153,917 |
| Total | $5,613,200 | $39,424,755 |
| Administrative costs | Annualized Value | Present Value |
|
$88,393 | $620,834 |
|
$2,435 | $17,102 |
| Total | $90,828 | $637,936 |
| Total cost (all impacted small businesses) | $5,704,028 | $40,062,691 |
"One-for-One" Rule
As per the requirements of the Red Tape Reduction Act and the Red Tape Reduction Regulations, the increase in administrative burden costs on all affected industry stakeholders has been estimated over a 10-year period (2019–2020 to 2028–2029) and discounted to 2012 using a 7% real discount rate. The "One-for-One" Rule applies since there is an incremental increase in administrative burden on business, and the proposal is considered an "IN" for the administrative burden under the Rule. The proposal amends an existing regulation (the Cannabis Regulations), resulting in no net increase or decrease in regulatory titles.
The incremental increase in administrative burden for industry resulting from the proposed amendments to the Regulations has been estimated at $352,876 PV over the 10-year period, in 2012 dollars (or $50,242 annually). The anticipated increase in administrative burden is mostly related to the additional record-keeping requirements to which licensed processors would be subject (including the proposed requirements to keep records pertaining to phytocannabinoid and terpene profiles, ingredients and the purpose of each ingredient).
Regulatory cooperation and alignment
International
The Cannabis Regulations are part of a new and unique approach to controlling the public health and public safety risks associated with cannabis on a national scale. Canada has actively engaged international partners to promote understanding of the overarching objectives of the Cannabis Act. As the non-medical use of cannabis is currently illegal at the national level in other Organisation for Economic Co-operation and Development (OECD) member states, opportunities for regulatory alignment are limited. Nevertheless, given the scope of this national initiative, the regulatory framework has been informed by best practices and lessons learned from other jurisdictions, including a number of U.S. states that have legalized and regulated cannabis for non-medical purposes. Health Canada will continue to engage with these jurisdictions in order to collect and share public health information and best practices in this field.
Canada is a party to three United Nations drug control conventions: the Single Convention on Narcotic Drugs, 1961 as amended by the 1972 Protocol; the Convention on Psychotropic Substances, 1971; and the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances of 1988. General international trade in cannabis is prohibited by the drug conventions of the United Nations. Therefore, under the Cannabis Act and its regulations, the import and export of cannabis is permitted only for scientific or medical purposes, or in respect of industrial hemp. A permit is required from the Minister in order to import or export cannabis. No changes to the import and export provisions of the Regulations are proposed.
Provinces and territories
Building on the advice provided by the Task Force, Health Canada has maintained regular and ongoing contact with the provinces and territories throughout the development of the legislative and regulatory frameworks at the federal level and in each jurisdiction. Given the provincial and territorial responsibility for distribution and retail sale of cannabis, provincial and territorial governments were engaged extensively throughout the development of the Regulations, and will be similarly engaged and consulted on the proposed amendments addressing the public health and public safety risks of edible cannabis, cannabis extracts, and cannabis topicals. Federal, provincial and territorial officials have worked closely together to design and implement the new legal and regulatory frameworks and are expected to continue to do so.
Following the publication of the proposed regulatory amendments in the Canada Gazette, Part I, Health Canada will hold bilateral meetings with representatives of all provincial and territorial governments to help ensure their perspectives and feedback are considered in the development of final Regulations. This is in addition to ongoing meetings with senior officials from all provinces and territories, which are held every three weeks.
Strategic environmental assessment
In accordance with the Cabinet Directive on the Environmental Assessment of Policy, Plan and Program Proposals, a preliminary scan was conducted; it was concluded that a detailed analysis is not required.
The proposed regulatory amendments are not expected to have any significant impacts with respect to key environmental indicators, such as emissions of greenhouse gases and air pollutants, or drinking water quality.
Based in part on feedback received from regulated parties, proposed amendments to existing packaging and labelling requirements under the Regulations (in particular, proposed amendments that would allow for the use of peel-back or accordion panels and labels that extend beyond the exterior display surface of the package) are expected to enable smaller package sizes for cannabis products without compromising the public health objectives of current packaging and labelling requirements (such as protecting the health of young persons by restricting their access to cannabis, protecting young persons and others from inducements to use cannabis, and promoting informed consumer choice). It is anticipated that these amendments would have a positive impact on the environment, in that they would result in an overall reduction in the use of packaging materials, and consequently a reduction in the overall volume of packaging waste.
Gender-based analysis plus
Prevalence of cannabis use according to age and sex
Canadian surveys of cannabis use suggest there are differences in the prevalence of cannabis use according to age (with youth and young adults having higher rates of use than adults over the age of 25) and sex (with males having higher rates of use than females). There is also evidence to suggest that males are more likely than females to use cannabis on a daily or almost daily basis.
According to the 2017 Canadian Tobacco, Alcohol and Drugs Survey (CTADS), footnote 20 a biennial general population survey of tobacco, alcohol and drug use among Canadians aged 15 years and older, 19% of youth (i.e. individuals aged 15 to 19) and 33% of young adults (i.e. individuals aged 20 to 24) reported past-year cannabis use. This prevalence is markedly higher than that of adults aged 25 and older, only 13% of whom reported past-year cannabis use. The prevalence of past-year cannabis use among males aged 15 years and older (19%) was higher than among females (11%). However, the prevalence of cannabis use among Canadian females aged 15 years and older was reported to be higher in 2017 (11%), as compared with a past-year use rate of 7% among females in 2013, footnote 21 but unchanged compared to 10% in 2015. footnote 22 Nonetheless, the age of initiation to cannabis use did not differ significantly by sex, at 18 years for males and 19 years for females. The average age of initiation is highly dependent on the age range of the respondents in the survey, as well as the distribution of respondents within predetermined age groups. Adults aged 25 years and older were on average 19 years old when they first tried cannabis, compared to 17 years among individuals aged 20 to 24 and 16 years among individuals aged 15 to 19.
The 2016–2017 Canadian Student Tobacco, Alcohol and Drugs Survey (CSTADS) found that 17% of respondents in grades 7 to 12 reported using cannabis in the last 12 months. Of those surveyed, 18% of males reported using cannabis, compared with 16% of females. footnote 23 Females were slightly older than males when they first used cannabis, at 14.4 years for females, compared to 14.1 years for males.
The 2018 Canadian Cannabis Survey footnote 24 (CCS) found that 25% of people who used cannabis in the past 12 months reported daily or almost daily use. A greater percentage of males (28%) reported daily or almost daily use compared to females (21%), whereas a greater percentage of females (43%) reported less than monthly use compared to males (30%).
Impacts of cannabis use according to sex and age
The current state of the evidence suggests sex-dependent differences with respect to a number of cannabis outcomes. For example, studies suggest a greater prevalence of problematic cannabis use (i.e. cannabis use disorder) among males. footnote 25, footnote 26 Other studies suggest females are more sensitive to the effects of THC, need less THC to achieve intoxication, and are more likely to experience adverse effects related to acute cannabis consumption. footnote 27 Evidence also suggests that female cannabis consumers progress more quickly to dependence/addiction. footnote 28, footnote 29, footnote 30, footnote 31 More research is needed to explain the fundamental sex-dependent differences relating to cannabis use.
Regardless of sex, the risks of experiencing adverse effects from cannabis appear to be greater if using cannabis products with a higher concentration of THC. footnote 32
Studies suggest that cannabis use during pregnancy is associated with a number of different negative outcomes for children, including low birth weight and poorer longer-term developmental outcomes. Cannabis use that begins early in adolescence, that is frequent and that continues over time has been associated with an increased risk of these harms. Some of these harms may not be fully reversible. footnote 33 Youth are particularly vulnerable to the effects of cannabis. This is because THC affects the same components in the brain that direct brain development, and research has shown that adolescence is a critical time for brain development. footnote 34
The studies above suggest that public education efforts should take sex and age-specific factors into consideration when developing key messages on effects and risks.
Patterns of cannabis use according to product class — Sex
Findings from the 2018 Canadian Cannabis Survey footnote 35 (CCS) suggest the most common type of cannabis product used is dried flower/leaf (82%), followed by edible cannabis (41%), hashish/kief (26%), solid concentrates (19%), liquid concentrates (17%), cannabis oil cartridges or disposable vape pens (16%), beverages (4%), and other products (4%). Use of hashish/kief was more common among males than females (31% among males, versus 19% among females). A similar pattern was observed for dried flower/leaf (83% of males, versus 80% of females), liquid concentrates (20% of males, versus 14% of females), solid concentrates (20% of males, versus 17% of females), and cannabis oil cartridges or disposable vape pens (18% of males, versus 14% of females). For edible cannabis, there was no significant difference in consumption between males (40%) and females (43%).
Patterns of cannabis use according to product class — Age
According to the 2018 CCS, respondents under the age of 25 reported greater use of dried cannabis, cannabis concentrates and extracts, and cannabis edibles than those aged 25 years and older. Specifically, the use of
- Dried leaf/flower was reported by 85% of those aged 16 to 19 years and 86% of those aged 20 to 24, versus 81% of those aged 25 and older;
- Hashish/kief was reported by 47% of those aged 16 to 19 years and 34% of those aged 20 to 24 years, versus 22% of those aged 25 and older;
- Liquid concentrates was reported by 22% of those aged 20 to 24, versus 17% of those aged 25 and above; and was unreportable for those aged 16 to 19 years;
- Solid concentrates was reported by 32% of those aged 16 to 19 years and 28% of those aged 20 to 24 years, versus 15% of those aged 25 and older; and
- Edible cannabis was reported by 43% of those aged 16 to 19 years and 50% of those aged 20 to 24 years, versus 39% of those aged 25 years and older.
Similar patterns of use were observed among students in grades 7 to 12 as part of the 2016–17 CSTADS. Among students in grades 7 to 12 who used cannabis, 80% reported smoking cannabis, 34% reported consuming edible forms of cannabis, 30% reported vaping, 22% reported dabbing cannabis, and 14% reported drinking cannabis. Approximately 25% of students who reported using cannabis reported using more than one method of consumption. footnote 36
Health Canada will continue to use national surveys such as the CCS, CTADS and CSTADS to monitor trends in cannabis use in Canada.
Indigenous considerations
Evidence suggests that Indigenous peoples are at a greater risk of experiencing complex mental health and substance use issues due to a variety of factors, including the intergenerational impacts of Indian Residential Schools and colonialism, which have had lasting effects on many communities and families, as well as social, economic, and cultural inequities that persist today. footnote 37 In addition, those living in rural and remote areas have an increased vulnerability to mental wellness challenges due to their isolation. footnote 38 The Regional Health Survey (RHS) is a cross-sectional survey of First Nations living on-reserve and in northern communities across Canada. According to Phase 3 of the RHS, footnote 39 past-year cannabis use among First Nations adults 18 years of age and older is 30%; approximately 12% of adults reported daily or almost daily use. Past-year use among youth aged 12–17 decreased from 36% in Phase 2 to 27% in Phase 3.
Recognizing the unique context, interests and priorities of First Nations, Inuit and Métis across Canada, Health Canada will continue to engage and collaborate with Indigenous leadership, organizations, and communities over the long term as the cannabis regulatory framework is implemented and evolves. This will include sharing information about legalization and regulation, ensuring public education and awareness activities are effective, and ensuring that their interests are being fully considered. Health Canada will also continue to work together with government partners such as Indigenous Services Canada and Crown-Indigenous Relations and Northern Affairs Canada to support Indigenous organizations with expertise in mental wellness and substance use to lead key engagement and public education activities, and to support those interested in economic development and partnerships in the legal cannabis industry.
Implementation, compliance and enforcement, and service standard
Implementation
Licence amendments and new product notifications
As mentioned earlier, it is proposed that a processing licence (either micro or standard) under the Regulations would be required in order to produce, package and label the proposed new classes of cannabis. Health Canada has adopted a graduated licensing approach, whereby licence holders may first be authorized to conduct an initial set of activities, and must obtain approval from Health Canada in order to conduct new activities (or the same activity in respect of another class of cannabis). For example, a licence holder may be authorized to sell certain classes of cannabis (e.g. dried, fresh) to provinces and territories or registered medical clients, but not other classes (e.g. oil). This has been operationalized through a condition of licence, which spells out any restrictions pertaining to the authorized activities.
As part of this graduated licensing approach, licensed processors must apply to Health Canada to amend their licence and demonstrate that they meet all of the regulatory requirements for the new classes of cannabis prior to being able to sell them.
In addition, as per section 244 of the Regulations, at least 60 days before making a new cannabis product available for sale, holders of a processing licence would need to provide Health Canada with a written notice that states the class of cannabis to which the product belongs, a description of the product (including the brand name), and the date on which the product is expected to be made available for sale. As is currently the case, notification of a new product would not constitute "approval" for sale by Health Canada. Licence holders would continue to be responsible for making sure the new product meets all of the requirements set out in the Act and Regulations.
Cannabis oil
One of the key changes proposed is that cannabis oil would be removed from Schedule 4 and would instead be subsumed under the other classes of cannabis. It is proposed that the amended Regulations would include transitional provisions to allow current regulated parties to continue their authorized activities with respect to cannabis oil while they put in place measures to meet the proposed new regulatory requirements. For example, it is proposed that there would be a six-month transition period following the coming into force of the amended Regulations for requirements pertaining to cannabis oil. During this time, cannabis oil products could continue to meet the requirements under the Regulations as they read before the amendments come into force. This transition period is intended to allow sufficient time for industry to make the necessary updates to its products, packages and labels to meet the new requirements, as well as to exhaust any existing stock of cannabis oil.
Communications and guidance
Health Canada is committed to continuing to provide industry, the provinces and territories, and other stakeholders with relevant and timely information. External guidance will be developed and updated to facilitate transition. Key information to enable a smooth transition will be provided to industry as early as possible.
Consistency with other regulatory frameworks
The proposed rules for edible cannabis, cannabis extracts, and cannabis topicals have been developed taking into consideration existing regulatory frameworks for food, vaping products, and cosmetics. Health Canada will continue to work with the Canadian Food Inspection Agency and regulatory programs within Health Canada in order to ensure that the proposed product rules are consistent with those under other frameworks, as appropriate, and remain consistent over time.
Compliance and enforcement
Health Canada will continue to provide oversight to verify that regulated parties are aware of and adhere to the proposed new regulatory requirements. Health Canada will take timely actions respecting individuals and businesses whose cannabis products or activities with cannabis pose an unacceptable risk to public health and/or public safety or do not comply with the applicable requirements. Health Canada's national compliance and enforcement approach would continue to apply, including promoting and verifying compliance with the Act and its regulations through inspections and other means, and working towards preventing non-compliance. In alignment with the Health Canada Compliance and enforcement policy framework and the Health Canada Compliance and Enforcement Policy for the Cannabis Act, and informed by the circumstances of each case, Health Canada takes a risk-based approach to its enforcement actions and will choose the most appropriate tool to achieve compliance and mitigate risks as circumstances warrant. The enforcement measures under the Cannabis Act and the Cannabis Regulations will continue to be available to Health Canada. These measures will maintain the same delivery approach, which ranges from activities intended to educate and prevent non-compliance through compliance promotion, to measures intended to correct non-compliance or address a risk to public health or public safety. Enforcement measures could include, but are not limited to, warnings, product recall, product seizure, placing conditions on a federal licence, suspending or revoking a federal licence or permit, issuing administrative monetary penalties of up to $1 million, ministerial orders, or prosecution.
To support its compliance objectives, Health Canada will also continue to collaborate with other partners, including the provinces and territories, law enforcement, the Canada Border Services Agency, the Canada Revenue Agency, the Canadian Food Inspection Agency and the Public Health Agency of Canada. Supported by the provisions in the Cannabis Act related to information disclosure, Health Canada may also disclose relevant information obtained under the Act where the disclosure is necessary to protect public health or public safety.
Service standards
Health Canada intends to continue to monitor its performance against administrative, non-binding service standards established to support the Cannabis Fees Order with respect to the screening of licence applications as well as to issuing import and export permits. The overall program administration will continue to be closely monitored with a view to establishing service standards in other areas, such as licence amendments. Health Canada is also committed to creating a forum to engage with the cannabis industry on the administration of the fee regime and as it develops additional administrative service standards, supporting predictability and transparency.
Public comment period
Any person may, within 60 days after publication of this notice, submit written comments on the proposals outlined herein. Comments can be sent by email to cannabis@canada.ca, or by mail to the contact information provided below. An online questionnaire is also available at www.canada.ca/cannabis.
Contact
Eric Costen
Director General
Strategic Policy
Cannabis Legalization and Regulation Branch
Address locator: 0302B
Health Canada
Ottawa, Ontario
K1A 0K9
Email: cannabis@canada.ca
PROPOSED REGULATORY TEXT
Notice is given that the Governor in Council, pursuant to subsection 139(1) of the Cannabis Act footnote a, proposes to make the annexed Regulations Amending the Cannabis Regulations (New Classes of Cannabis).
Interested persons may make representations concerning the proposed regulations within 60 days after the date of publication of this notice. All such representations must cite the Canada Gazette, Part I, and the date of publication of this notice, and be addressed to Eric Costen, Director General, Strategic Policy, Cannabis Legalization and Regulation Branch, Address locator: 0302B, Health Canada, Ottawa, Ontario K1A 0K9 (email: cannabis@canada.ca).
Ottawa, December 6, 2018
Jurica Čapkun
Assistant Clerk of the Privy Council
Regulations Amending the Cannabis Regulations (New Classes of Cannabis)
Amendments
1 (1) The definitions cannabis non-solid concentrates and cannabis solid concentrates in subsection 1(1) of the Cannabis Regulations footnote 40 are repealed.
(2) The definition cannabis oil in subsection 1(1) of the Regulations is repealed.
(3) Subsection 1(1) of the Regulations is amended by adding the following definitions in alphabetical order:
cannabis concentrate means a substance that has a maximum yield percentage of greater than 3% w/w of THC, taking into account the potential to convert THCA into THC. (cannabis sous forme d'un concentré)
cannabis extract means, with the exception of cannabis topicals and edible cannabis,
- (a) a substance that has been produced by
- (i) subjecting anything referred to in item 1 of Schedule 1 to the Act to extraction processing, or
- (ii) synthesizing a substance that is identical to a phytocannabinoid produced by, or found in, a cannabis plant; or
- (b) a substance or mixture of substances that contains or has on it a substance that has been produced in a manner referred to in paragraph (a). (extrait de cannabis)
cannabis topical means a substance or mixture of substances that contains or has on it anything referred to in item 1 or 3 of Schedule 1 to the Act and that is intended for use, directly or indirectly, exclusively on external body surfaces, including hair and nails. (cannabis pour usage topique)
edible cannabis means a substance or mixture of substances that contains or has on it anything referred to in item 1 or 3 of Schedule 1 to the Act and that is intended to be consumed in the same manner as food. It does not include dried cannabis, fresh cannabis, cannabis plants or cannabis plant seeds. (cannabis comestible)
(4) The definition common name in subsection 1(2) of the Regulations is repealed.
(5) The definition cannabis product in subsection 1(2) of the Regulations is replaced by the following:
cannabis product means cannabis of only one of the classes set out in Schedule 4 to the Act — or a cannabis accessory that contains such cannabis — after it has been packaged and labelled for sale to a consumer at the retail level. It does not include
- (a) cannabis that is intended for an animal;
- (b) a cannabis accessory that contains cannabis that is intended for an animal; or
- (c) a drug containing cannabis. (produit du cannabis)
(6) Subsection 1(2) of the Regulations is amended by adding the following definitions in alphabetical order:
constituent means an individual unit of food that is combined as an individual unit of food with one or more other individual units of food to form an ingredient. (constituant)
contaminated means, in respect of cannabis or an ingredient, the presence of anything — including a micro-organism but excluding anything referred to in item 1 or 3 of Schedule 1 to the Act — that may render the cannabis or ingredient injurious to human health or unsuitable for human use. (contaminé)
durable life means the period, commencing on the day on which a cannabis product is packaged for sale to consumers at a retail level, during which the product, when it is stored under conditions appropriate to that product, will retain, without any appreciable deterioration, normal palatability, and any other qualities claimed for it by the holder of a licence for processing that manufactured the product. (durée de conservation)
durable life date means the date on which the durable life of a cannabis product ends. (date limite de conservation)
food has the same meaning as in section 2 of the Food and Drugs Act. (aliment)
food additive means any substance the use of which results, or may reasonably be expected to result, in it or its by-products becoming a part of, or affecting the characteristics of, a food or edible cannabis but does not include
- (a) anything referred to in item 1 or 3 of Schedule 1 to the Act; or
- (b) anything that is excluded from the definition food additive in subsection B.01.001(1) of the Food and Drug Regulations. (additif alimentaire)
immediate container means the container that is in direct contact with cannabis that is a cannabis product or — if a wrapper is in direct contact with the cannabis — with the wrapper. (contenant immédiat)
ingestion includes absorption in the mouth. (ingestion)
ingredient means
- (a) in the case of a cannabis extract or cannabis topical, a substance, other than a substance referred to in item 1 or 3 of Schedule 1 to the Act, that is intended to be present in the final form of the cannabis extract or cannabis topical; and
- (b) in the case of edible cannabis,
- (i) a substance, other than a substance that is referred to in item 1 or 3 of Schedule 1 to the Act,
- (A) that is used to make the edible cannabis where the use results, or may reasonably be expected to result, in the substance or its by-products becoming a part of, or affecting the characteristics of, the edible cannabis, or
- (B) that is part of a mixture of substances referred to in item 2 of the Schedule that is used to make the edible cannabis where the use results, or may reasonably be expected to result, in the substance or its by-products becoming a part of, or affecting the characteristics of, the edible cannabis, or
- (ii) a mixture of substances, other than a mixture of substances that are referred to in item 1 or 3 of Schedule 1 to the Act,
- (A) that is used to make the edible cannabis where the use results, or may reasonably be expected to result, in the mixture or its by-products becoming a part of, or affecting the characteristics of, the edible cannabis, or
- (B) that is part of a mixture of substances referred to in item 2 of the Schedule that is used to make the edible cannabis where the use results, or may reasonably be expected to result, in the first mixture or its by-products becoming a part of, or affecting the characteristics of, the edible cannabis. (ingrédient)
- (i) a substance, other than a substance that is referred to in item 1 or 3 of Schedule 1 to the Act,
pest control product has the same meaning as in subsection 2(1) of the Pest Control Products Act. (produit antiparasitaire)
sugars has the same meaning as in subsection B.01.001(1) of the Food and Drug Regulations. (sucres)
(7) Section 1 of the Regulations is amended by adding the following after subsection (3):
Deeming — immediate container
(4) For the purposes of these Regulations, a cannabis accessory that contains edible cannabis in liquid form at a temperature of 22 ± 2°C and that is a cannabis product is deemed to be an immediate container.
2 Subsection 4(4) of the Regulations is replaced by the following:
Authorized activities — accredited laboratory
(4) Individuals who are involved in the testing of cannabis as a requirement of their duties at a laboratory that is designated as an accredited laboratory under section 2.1 of the Seeds Act are authorized to conduct the activities referred to in paragraphs (1)(a), (c) and (d), and to offer to conduct the activity referred to in paragraph (1)(c), to the extent necessary to conduct the testing.
3 The Regulations are amended by adding the following after section 5:
Sale of cannabis containing caffeine
5.1 For the purposes of subsection 34(1) of the Act,
- (a) a holder of a licence for processing that authorizes the sale of cannabis may, in accordance with the licence, sell edible cannabis that is not a cannabis product and that contains caffeine if the caffeine has been introduced through the use of ingredients that naturally contain caffeine; and
- (b) the following persons may, in accordance with their licence or the provincial authorization, as the case may be, sell edible cannabis that is a cannabis product and that contains caffeine if the caffeine has been introduced through the use of ingredients that naturally contain caffeine and the total amount of caffeine in each immediate container of the cannabis product does not exceed 30 mg:
- (i) a holder of a licence for processing that authorizes the sale of cannabis,
- (ii) a holder of a licence for sale that authorizes the sale of cannabis products, or
- (iii) a person that is authorized under a provincial Act referred to in subsection 69(1) of the Act to sell cannabis.
Sale of cannabis containing ethyl alcohol
5.2 (1) For the purposes of subsection 34(1) of the Act,
- (a) a holder of a licence for processing that authorizes the sale of cannabis may, in accordance with the licence, sell a cannabis extract that is not a cannabis product and that contains ethyl alcohol; and
- (b) the following persons may, in accordance with their licence or the provincial authorization, as the case may be, sell a cannabis extract that is a cannabis product and that contains ethyl alcohol if the cannabis extract is intended to be ingested and the net weight of the cannabis extract in each immediate container of the cannabis product does not exceed 7.5 g:
- (i) a holder of a licence for processing that authorizes the sale of cannabis,
- (ii) a holder of a licence for sale that authorizes the sale of cannabis products, or
- (iii) a person that is authorized under a provincial Act referred to in subsection 69(1) of the Act to sell cannabis.
Edible cannabis
(2) For the purposes of subsection 34(1) of the Act,
- (a) a holder of a licence for processing that authorizes the sale of cannabis may, in accordance with the licence, sell edible cannabis that is not a cannabis product and that contains ethyl alcohol; and
- (b) the following persons may, in accordance with their licence or the provincial authorization, as the case may be, sell edible cannabis that is a cannabis product and that contains ethyl alcohol if the concentration of ethyl alcohol does not exceed 0.5% w/w of the edible cannabis:
- (i) a holder of a licence for processing that authorizes the sale of cannabis,
- (ii) a holder of a licence for sale that authorizes the sale of cannabis products, or
- (iii) a person that is authorized under a provincial Act referred to in subsection 69(1) of the Act to sell cannabis.
4 Subsection 10(1) of the Regulations is replaced by the following:
Obtaining cannabis
10 (1) Subject to the other provisions of these Regulations, a holder of a licence that authorizes the possession of cannabis must only possess cannabis that was obtained in accordance with the former Access to Cannabis for Medical Purposes Regulations, or that is obtained in accordance with these Regulations or from a person authorized by subsection 69(1) of the Act to sell cannabis.
5 (1) Subparagraph 11(5)(c)(ii) of the Regulations is replaced by the following:
- (ii) a person authorized by subsection 69(1) of the Act to sell cannabis; and
(2) Subparagraph 11(5)(d)(i) of the Regulations is replaced by the following:
- (i) a person authorized by subsection 69(1) of the Act to sell cannabis, or
6 (1) Subparagraph 14(5)(b)(ii) of the Regulations is replaced by the following:
- (ii) a person authorized by subsection 69(1) of the Act to sell cannabis; and
(2) Subparagraph 14(5)(c)(i) of the Regulations is replaced by the following:
- (i) a person authorized by subsection 69(1) of the Act to sell cannabis, or
7 (1) Subparagraph 17(5)(d)(ii) of the Regulations is replaced by the following:
- (ii) a person authorized by subsection 69(1) of the Act to sell cannabis; and
(2) Subparagraph 17(5)(e)(i) of the Regulations is replaced by the following:
- (i) a person authorized by subsection 69(1) of the Act to sell cannabis, or
(3) Subsection 17(6) of the English version of the Regulations is replaced by the following:
Client's shipping address
(6) If a holder of a licence for micro-processing or standard processing sends or delivers cannabis products under subparagraph (5)(e)(ii) further to the sale of such products under section 289, the holder must send or deliver the products to the client's shipping address as indicated by the holder of a licence for sale for medical purposes.
8 Paragraph 18(1)(a) of the Regulations is replaced by the following:
- (a) the sale and distribution of cannabis products to a person authorized by subsection 69(1) of the Act to sell cannabis; and
9 (1) Subsection 19(1) of the Regulations is replaced by the following:
Quality assurance person
19 (1) A holder of a licence for processing must retain the services of one individual as a quality assurance person who has the training, experience and technical knowledge related to the requirements of Parts 5 and 6 that are applicable to the class of cannabis in respect of which activities are conducted under the licence.
Exception — edible cannabis
(1.1) Despite subsection (1), if the quality assurance person does not have the training, experience and technical knowledge related to the requirements of Parts 5 and 6 that are applicable to edible cannabis, the holder of a licence for processing that conducts activities in respect of that class of cannabis must retain the services of another individual who has that training, experience and technical knowledge.
(2) Subsection 19(2) of the Regulations is amended by striking out "and" at the end of paragraph (a) and by replacing paragraph (b) with the following:
- (b) investigating every complaint received in respect of the quality of the cannabis and, if necessary, immediately taking measures to mitigate any risk; and
- (c) in the case where they suspect, on reasonable grounds, that the cannabis or anything that will be used as an ingredient presents a risk of injury to human health or does not meet the requirements of Part 5 or 6, immediately investigating the matter and, if necessary, immediately taking measures to mitigate the risk.
| Item | Column 1 Class of cannabis |
Column 2 Amount that is equivalent to 1 kg of dried cannabis |
|---|---|---|
| 5 | cannabis concentrates | 0.25 kg |
11 Section 22 of the Regulations is amended by adding the following after subsection (3):
Distribution
(4) A holder of a licence for analytical testing is also authorized, for the purpose of testing, to distribute cannabis to another holder of a licence for analytical testing.
12 Subsection 23(1) of the Regulations is replaced by the following:
Head of laboratory
23 (1) A holder of a licence for analytical testing must retain the services of one individual as the head of laboratory who must work at the site set out in the licence and who is responsible for the testing referred to in sections 90 to 91.1.
13 Section 29 of the Regulations is amended by striking out "and" at the end of paragraph (i), by adding "and" at the end of paragraph (j) and by adding the following after paragraph (j):
- (k) in respect of a licence for processing, the applicant has, in the past 10 years, been convicted of an offence under the Safe Food for Canadians Act or an Act referred to in subsection 374(2) of the Safe Food for Canadians Regulations.
14 Section 31 of the Regulations is amended by striking out "and" at the end of paragraph (c), by adding "and" at the end of paragraph (d) and by adding the following after paragraph (d):
- (e) in the case of a licence for processing, the holder has, since its issuance, been convicted of an offence under the Safe Food for Canadians Act or an Act referred to in subsection 374(2) of the Safe Food for Canadians Regulations.
15 Section 42 of the Regulations is renumbered as subsection 42(1) and is amended by adding the following after that subsection:
Irradiation of edible cannabis
(2) For greater certainty, the conditions set out in paragraphs 102.6(a) and (b) also apply in the case of the irradiation of edible cannabis by a holder of a licence for processing.
16 Section 46 of the Regulations is renumbered as subsection 46(1) and is amended by adding the following after that subsection:
Recall simulation
(2) The holder must
- (a) at least once every 12 months, conduct a recall simulation based on the system of control;
- (b) after completing the recall simulation, prepare a document that sets out the details of how it was conducted and the results; and
- (c) retain the document for at least two years after the day on which the recall simulation is completed.
17 The heading before section 79 and sections 79 and 80 of the Regulations are replaced by the following:
Definitions
Definitions
78.1 The following definitions apply in this Part.
acceptable level means a level of a biological, chemical or physical hazard that does not present a risk of contamination of the cannabis or anything that will be used as an ingredient. (niveau acceptable)
control measure means a measure that can be applied to prevent or eliminate any biological, chemical or physical hazard that presents a risk of contamination of the cannabis or anything that will be used as an ingredient, or to reduce the hazard to an acceptable level. (mesure de contrôle)
critical control point means a step at which the application of a control measure is essential to prevent or eliminate any biological, chemical or physical hazard that presents a risk of contamination of the cannabis or anything that will be used as an ingredient, or to reduce the hazard to an acceptable level. (point de contrôle critique)
sanitary condition means a condition that does not present a risk of contamination, allergen cross-contamination or introduction of an extraneous substance to the cannabis or anything that will be used as an ingredient. (conditions hygiéniques)
General Requirements
Application
79 The requirements of this Part only apply to activities that the holder of a licence conducts in respect of cannabis or a thing that will be used as an ingredient.
Sale, distribution and exportation — cannabis
79.1 A holder of a licence must not sell, distribute or export cannabis unless the holder meets the requirements set out in sections 80 to 88.94 that apply to the activities that they conduct.
Standard operating procedures
80 Cannabis and anything that will be used as an ingredient must be produced, packaged, labelled, distributed, stored, sampled and tested in accordance with standard operating procedures that are designed to ensure that those activities are conducted in accordance with the requirements of this Part and Part 6.
18 Sections 82 to 85 of the Regulations are replaced by the following:
Sanitizers, agronomic inputs and non-food chemical agents
81.1 Any sanitizer, agronomic input or non-food chemical agent that is present at a site must
- (a) be properly and clearly identified;
- (b) be suitable for its intended use and not present a risk of contamination of the cannabis or anything that will be used as an ingredient; and
- (c) be handled and used in a manner that does not present a risk of contamination of the cannabis or anything that will be used as an ingredient and that is in accordance with any manufacturer's instructions.
Storage
82 Cannabis and anything that will be used as an ingredient must be stored under conditions that maintain its quality.
Distribution
83 Cannabis and anything that will be used as an ingredient must be distributed in a manner that maintains its quality.
Building or part of building
84 Any building or part of a building where cannabis or anything that will be used as an ingredient is produced, packaged, labelled, stored or tested must be designed, constructed and maintained in a manner that permits those activities to be conducted appropriately and under sanitary conditions and, in particular, that
- (a) permits the building or part of the building to be kept clean and orderly;
- (b) permits the effective cleaning of all surfaces in the building or part of the building;
- (c) prevents the contamination of the cannabis or anything that will be used as an ingredient; and
- (d) prevents the addition of an extraneous substance to the cannabis or anything that will be used as an ingredient.
Ventilation system
85 Any building or part of a building where cannabis or anything that will be used as an ingredient is produced, packaged, labelled, stored or tested must be equipped with a ventilation system that
- (a) prevents the escape of cannabis odours from the building or part of the building, as the case may be;
- (b) provides sufficient air exchange to provide clean air and to remove unclean air and odours in order to prevent the contamination of the cannabis or anything that will be used as an ingredient;
- (c) is accessible and, if necessary for its cleaning, maintenance or inspection, is able to be disassembled;
- (d) is capable of withstanding repeated cleaning; and
- (e) functions in accordance with its intended use.
Supply of water
85.1 Any system that supplies water to a site must be adequate to the activities being conducted and any system that supplies potable water to a site must not be cross-connected with any other system, unless measures are taken to eliminate any risk of contamination of the cannabis or anything that will be used as an ingredient as a result of the cross-connection.
Lighting
85.2 (1) Any building or part of a building where cannabis or anything that will be used as an ingredient is produced, packaged, labelled, stored or tested must be equipped with natural or artificial lighting that is appropriate for the activity being conducted.
Light fixtures
(2) Any light fixtures in the building or part of the building where the activities referred to in subsection (1) are conducted must
- (a) be capable of withstanding repeated cleaning and, if necessary to prevent contamination of the cannabis or the thing that will be used as an ingredient, repeated sanitizing; and
- (b) not present a risk of contamination of the cannabis or the thing that will be used as an ingredient in the event of breakage.
19 (1) The portion of subsection 86(1) of the Regulations before paragraph (a) is replaced by the following:
Equipment
86 (1) Cannabis and anything that will be used as an ingredient must be produced, packaged, labelled, stored, sampled and tested using equipment that is designed, constructed, maintained, operated and arranged in a manner that
(2) Paragraphs 86(1)(c) and (d) of the Regulations are replaced by the following:
- (b.1) is accessible and, if necessary for its cleaning, maintenance or inspection, is able to be disassembled;
- (c) prevents the contamination of the cannabis or anything that will be used as an ingredient;
- (d) prevents the addition of an extraneous substance to the cannabis or anything that will be used as an ingredient; and
- (e) protects the cannabis or anything that will be used as an ingredient against allergen cross-contamination.
(3) Subsection 86(2) of the Regulations is replaced by the following:
Conveyances
(1.1) Cannabis and anything that will be used as an ingredient must be distributed using a conveyance that is designed, constructed, maintained and operated in a manner that prevents the contamination of the cannabis or anything that will be used as an ingredient.
Non-application
(2) Paragraphs (1)(d) and (e) do not apply to the outdoor cultivation, propagation or harvesting of cannabis.
20 (1) The portion of subsection 87(1) of the Regulations before paragraph (a) is replaced by the following:
Sanitation program
87 (1) Cannabis and anything that will be used as an ingredient must be produced, packaged, labelled, distributed, stored, sampled and tested in accordance with a sanitation program that sets out
(2) Paragraph 87(1)(b) of the Regulations is replaced by the following:
- (b) procedures for effectively cleaning the equipment and conveyances used in those activities;
21 Section 88 of the Regulations is replaced by the following:
Hand cleaning and sanitizing stations and lavatories
87.1 (1) If necessary to prevent the contamination of cannabis or anything that will be used as an ingredient, the building or part of the building where cannabis or anything that will be used as an ingredient is produced, packaged, labelled, stored and tested must be equipped with hand cleaning and sanitizing stations and lavatories that
- (a) are appropriately equipped and adequate in number and size for the number of individuals using them;
- (b) are located so that they are readily accessible to the individuals using them; and
- (c) are capable of withstanding repeated cleaning and, if necessary to prevent contamination of the cannabis or the thing that will be used as an ingredient, repeated sanitizing.
Hand cleaning and sanitizing stations
(2) The hand cleaning and sanitizing stations must permit the effective cleaning and sanitation of hands.
Additional requirements — Holder of a licence for processing
Quality assurance
88 A holder of a licence for processing must ensure that
- (a) every investigation in respect of the matters referred to in paragraph 19(2)(b) or (c) is conducted by the quality assurance person referred to in section 19;
- (b) if necessary following an investigation, the quality assurance person immediately takes measures to mitigate any risk;
- (c) cannabis or anything that will be used as an ingredient is produced, packaged, labelled, distributed, stored, sampled and tested using methods and procedures that, prior to their implementation, have been approved by the quality assurance person;
- (d) in the case of a cannabis extract or edible cannabis, the quality assurance person approves the preventive control plan prior to its implementation; and
- (e) every lot or batch of cannabis is approved by the quality assurance person before it is made available for sale.
Competencies and qualifications
88.1 A holder of a licence for processing must ensure that any individual that conducts activities in relation to edible cannabis or anything that will be used as an ingredient in the production of edible cannabis has the competencies and qualifications that are necessary to conduct those activities at the site set out in the licence.
Temperature and humidity
88.2 (1) A holder of a licence for processing must ensure that the temperature and humidity level of a building or part of the building where cannabis or anything that will be used as an ingredient is produced, packaged, labelled, stored or tested is maintained at levels that are appropriate for the cannabis and the thing that will be used as an ingredient, as the case may be, and for the activity being conducted.
Heating, cooling or humidity-control system
(2) If the building or part of the building is equipped with a heating, cooling or humidity-control system, the holder must ensure that the system
- (a) if necessary to prevent contamination of the cannabis or anything that will be used as an ingredient, is equipped with instruments to control and indicate the temperature and humidity levels;
- (b) is accessible and, if necessary for its cleaning, maintenance or inspection, is able to be disassembled;
- (c) is capable of withstanding repeated cleaning; and
- (d) functions in accordance with its intended use.
Incompatible activities
88.3 (1) A holder of a licence for processing must ensure that physical or other effective means are used to separate incompatible activities in order to prevent contamination of the cannabis or anything that will be used as an ingredient.
Manufacture of food
(2) A holder of a licence for processing must not produce, package, label or store edible cannabis at a site set out in the licence if food that is for retail sale is also manufactured at that site.
Exception
(3) Despite subsection (2), edible cannabis may be produced, packaged, labelled and stored in a building within a site where food for retail sale is manufactured if food is not manufactured in the same building.
Separation of cannabis and ingredients from contaminants
88.4 A holder of a licence for processing must ensure that physical or other effective means are used to separate cannabis or anything that will be used as an ingredient from anything that presents a risk of contamination of the cannabis or the thing that will be used as an ingredient.
Ingredients — risk of injury to human health
88.5 (1) A holder of a licence for processing that produces edible cannabis must ensure that anything that will be used as an ingredient that presents a risk of injury to human health is identified as such and placed in a designated area when it arrives at the building or part of the building.
Measures to prevent contamination
(2) The holder of the licence must take any measures that are necessary to prevent the ingredient referred to in subsection (1) from contaminating the cannabis or any other thing that will be used as an ingredient.
Potable water
88.6 (1) A holder of a licence for processing must ensure that any water that might come into contact with a cannabis extract, cannabis topical, edible cannabis or anything that will be used as an ingredient is potable, unless it does not present a risk of contamination of the cannabis extract, cannabis topical, edible cannabis or anything that will be used as an ingredient.
Steam and ice from potable water
(2) A holder of a licence for processing must ensure that any steam or ice that might come into contact with a cannabis extract, cannabis topical, edible cannabis or anything that will be used as an ingredient is made from water that meets the requirements of subsection (1), unless the steam or ice does not present a risk of contamination of the cannabis extract, cannabis topical, edible cannabis or anything that will be used as an ingredient.
Animals — risk of contamination
88.7 (1) A holder of a licence for processing must ensure that any building or part of a building where cannabis or anything that will be used as an ingredient is produced, packaged, labelled or stored is protected against the entry of any animal that presents a risk of contamination of the cannabis or thing that will be used as an ingredient.
No presence of animals
(2) A holder of a licence for processing that produces edible cannabis must ensure that no animal is present in a building or part of a building where cannabis or anything that will be used as an ingredient in the production of edible cannabis is produced, packaged, labelled or stored.
Land — risk of contamination
88.8 If any land that forms part of a site set out in a licence for processing, or any land that is located near such a site, presents a risk of contamination of cannabis or anything that will be used as an ingredient, the holder of the licence must take measures to eliminate the risk.
Removal and disposal of contaminated materials and waste
88.9 (1) A holder of a licence for processing must ensure that there are means for the removal and disposal of contaminated materials and waste from the building or part of the building and, if necessary to prevent contamination of the cannabis or anything that will be used as an ingredient, that the building or part of the building is equipped with a drainage, sewage and plumbing system that functions in accordance with its intended use.
Frequency and manner
(2) The holder of the licence must ensure that contaminated materials and waste are removed and disposed of at a frequency that is sufficient to prevent contamination of the cannabis or anything that will be used as an ingredient and in a manner that does not present a risk of contamination of the cannabis or anything that will be used as an ingredient.
Conveyances and equipment
88.91 A holder of a licence for processing must ensure that any conveyance or equipment used on the site set out in the licence to handle any contaminated materials or any waste, unless that conveyance or equipment does not come into contact with those materials or waste,
- (a) be used only for that purpose;
- (b) be identified as being reserved for that purpose; and
- (c) meet the applicable requirements of section 86.
Clothing, footwear and protective coverings
88.92 A holder of a licence for processing must ensure that any individual who enters or is in an area of a building or part of a building where cannabis or anything that will be used as an ingredient is produced, packaged, labelled, stored, sampled or tested must wear clothing, footwear and protective coverings, including gloves, a hairnet, a beard net and a smock, that are in good condition, clean and in sanitary condition and that are appropriate for the cannabis, anything that will be used as an ingredient and for the activity being conducted.
Identification and analysis of hazards
88.93 (1) A holder of a licence for processing that produces a cannabis extract or edible cannabis must identify and analyze the biological, chemical and physical hazards that present a risk of contamination of the cannabis or anything that will be used as an ingredient in the production of the cannabis extract or edible cannabis.
Prevention, elimination and reduction of hazards
(2) The holder of the licence must prevent, eliminate or reduce to an acceptable level the hazards referred to in subsection (1) by using control measures that are shown by evidence to be effective, including any treatment or process.
Preventive control plan
88.94 (1) A holder of a licence for processing that conducts activities in relation to a cannabis extract or edible cannabis must prepare, retain, maintain and implement a written preventive control plan for any activity they conduct in respect of the cannabis or anything that will be used as an ingredient in the production of the cannabis extract or edible cannabis.
Content of preventive control plan
(2) The preventive control plan must include
- (a) a description of the measures for ensuring that the applicable requirements of sections 101.3, 101.4, 102, 102.2, 102.3, 102.5 and 102.6 are met;
- (b) in relation to the applicable requirements of these Regulations,
- (i) a description of the biological, chemical and physical hazards that are identified under subsection 88.93(1) that present a risk of contamination of the cannabis extract, edible cannabis or anything that will be used as an ingredient in the production of the cannabis extract or edible cannabis,
- (ii) a description of the control measures for preventing or eliminating the hazards referred to in subparagraph (i) or reducing them to an acceptable level and the evidence that the control measures are effective,
- (iii) a description of the critical control points, of the related control measures and of the evidence that the control measures are effective,
- (iv) a description of the critical limits for each critical control point,
- (v) the procedures for monitoring the critical control points in relation to their critical limits,
- (vi) the corrective action procedures for each critical control point,
- (vii) the procedures for verifying that the implementation of the preventive control plan results in compliance with these Regulations, and
- (viii) documents that substantiate that the preventive control plan has been implemented with respect to subparagraphs (i) to (vii); and
- (c) supporting documents that show evidence of the information recorded under paragraphs (a) and subparagraphs (b)(i) to (vii).
Retention period
(3) Each document referred to in subparagraph (2)(b)(viii) must be retained for at least two years after the day on which it is prepared.
22 Sections 90 to 92 of the Regulations are replaced by the following:
Testing for phytocannabinoids
90 (1) Testing for the quantity or concentration, as the case may be, of THC, THCA, CBD and CBDA must be conducted on each lot or batch of cannabis, other than cannabis plants or cannabis plant seeds, that
- (a) is or will become a cannabis product; or
- (b) is or will be contained in a cannabis accessory that is or will become a cannabis product.
Timing of testing
(2) The testing must be conducted on the final form of the cannabis, either before or after it — or the cannabis accessory that contains it — is packaged and labelled as a cannabis product.
Testing for contaminants
91 (1) Testing for microbial and chemical contaminants — other than residues of a pest control product or its components or derivatives — must be conducted on
- (a) each lot or batch of cannabis — other than cannabis plants or cannabis plant seeds — that
- (i) is or will become a cannabis product, or
- (ii) is or will be contained in a cannabis accessory that is or will become a cannabis product; or
- (b) each lot or batch of cannabis — other than cannabis plant seeds — that is used to produce cannabis referred to in paragraph (a).
Timing of testing
(2) Testing on
- (a) a lot or batch of cannabis referred to in paragraph (1)(a) must be conducted on the final form of the cannabis, either before or after it — or the cannabis accessory that contains it — is packaged and labelled as a cannabis product; and
- (b) a lot or batch of cannabis referred to in paragraph (1)(b) must be conducted after the final step in the production process during which the contaminants could be concentrated.
Tolerance limits
(3) The results of the testing referred to in subsection (1) must enable a determination of whether the contaminants, if any, are within the tolerance limits referred to in subsection 93(3) or 94(2) or section 101.1, as the case may be.
Dissolution and disintegration testing
91.1 (1) If cannabis, or a cannabis accessory that contains cannabis, is or will become a cannabis product to which subsection 95(1) applies, testing must be conducted on each lot or batch of the cannabis or cannabis accessory to demonstrate that the requirements referred to in that subsection are, or will be, met.
Timing of testing
(2) The testing must be conducted on the final form of the cannabis or cannabis accessory, either before or after it is packaged and labelled as a cannabis product.
Testing procedure
92 (1) Testing that is conducted under sections 90 to 91.1 — or to verify that the requirements under Part 6 are, or will be, met — must be conducted using validated methods on a representative sample of each lot or batch of cannabis or cannabis accessory.
Quantity
(2) A portion of the sample referred to in subsection (1) must be retained for at least one year after the date of the last sale of any portion of the lot or batch and must be of sufficient quantity to enable a determination of
- (a) whether the lot or batch meets the requirements of section 81 and the applicable requirements in Part 6; and
- (b) the quantity or concentration of THC, THCA, CBD, and CBDA.
23 Sections 93 to 98 of the Regulations are replaced by the following:
Interpretation — residues of pest control product
92.1 In this Part, a reference to residues of a pest control product includes the residues of any component or derivative of the pest control product.
Residues of pest control products — cannabis plants and seeds
92.2 Cannabis plants, or cannabis plant seeds, that are cannabis products — or that are contained in cannabis accessories that are cannabis products — must not contain or have on them residues of a pest control product that is registered for use on cannabis under the Pest Control Products Act, or that is otherwise authorized for use under that Act, unless the residues are within any maximum residue limits that are specified in relation to cannabis under section 9 or 10 of that Act.
Dried and fresh cannabis
93 (1) Dried or fresh cannabis that is a cannabis product — or that is contained in a cannabis accessory that is a cannabis product — must not contain or have on it anything other than a thing referred to in item 1 of Schedule 1 to the Act.
Residues of pest control products
(2) Despite subsection (1), cannabis that is referred to in that subsection may contain or have on it residues of a pest control product that is registered for use on cannabis under the Pest Control Products Act, or that is otherwise authorized for use under that Act, if the residues are within any maximum residue limits that are specified in relation to cannabis under section 9 or 10 of that Act.
Microbial and chemical contaminants
(3) Despite subsection (1), cannabis that is referred to in that subsection may contain or have on it microbial or chemical contaminants if the contaminants are within generally accepted tolerance limits for human use that are
- (a) established in a publication referred to in Schedule B to the Food and Drugs Act; and
- (b) appropriate for the intended use and any reasonably foreseeable use of the cannabis product.
More stringent limit applies
(4) If there are generally accepted tolerance limits referred to in subsection (3) that apply in respect of the residues of a pest control product referred to in subsection (2) for which a maximum residue limit has been specified in relation to cannabis under the Pest Control Products Act, the more stringent limit applies.
Cannabis used in production
94 (1) Cannabis that is referred to in item 1 or 3 of Schedule 1 to the Act and that is used in the production of the following cannabis must not contain or have on it residues of a pest control product that is registered for use on cannabis under the Pest Control Products Act, or that is otherwise authorized for use under that Act, unless the residues are within any maximum residue limits that are specified in relation to cannabis under section 9 or 10 of that Act:
- (a) a cannabis extract that will become a cannabis product or that will be contained in a cannabis accessory that will become a cannabis product;
- (b) a cannabis topical that will become a cannabis product or that will be contained in a cannabis accessory that will become a cannabis product; and
- (c) edible cannabis that will become a cannabis product or that will be contained in a cannabis accessory that will become a cannabis product.
Edible cannabis — microbial and chemical contaminants
(2) Cannabis that is referred to in item 1 or 3 of Schedule 1 to the Act and that is used in the production of edible cannabis must not, if the edible cannabis will become a cannabis product or will be contained in a cannabis accessory that will become a cannabis product, contain or have on it microbial or chemical contaminants unless the contaminants are within generally accepted tolerance limits for human use that are
- (a) established in a publication referred to in Schedule B to the Food and Drugs Act; and
- (b) appropriate for a product that is to be ingested.
Dissolution and disintegration
95 (1) Each discrete unit of a cannabis product that is intended for ingestion or nasal, rectal or vaginal use must, if the form of the unit is similar to a dosage form for which a dissolution or disintegration test is set out in a publication referred to in Schedule B to the Food and Drugs Act, meet the requirements of the test or, if there is more than one applicable test, the requirements of any such test that is suitable for demonstrating that the cannabis product will perform as intended.
Exception
(2) Subsection (1) does not apply in respect of edible cannabis.
Maximum yield quantity — discrete unit
96 (1) Each discrete unit of a cannabis product that is intended for ingestion or nasal, rectal or vaginal use must not exceed a maximum yield quantity of 10 mg of THC, taking into account the potential to convert THCA into THC.
Exception
(2) Subsection (1) does not apply in respect of edible cannabis.
Variability limits
97 (1) A cannabis extract, or cannabis topical, that is a cannabis product — or that is contained in a cannabis accessory that is a cannabis product — must not, in respect of any quantity or concentration of THC or CBD that is displayed on the label, contain less than 85% or more than 115% of that quantity or concentration.
Edible cannabis
(2) Edible cannabis that is a cannabis product — or that is contained in a cannabis accessory that is a cannabis product — must not
- (a) if a quantity of THC or CBD that is displayed on the label exceeds 5 mg, contain less than 85% or more than 115% of that quantity;
- (b) if a quantity of THC or CBD that is displayed on the label exceeds 2 mg but does not exceed 5 mg, contain less than 80% or more than 120% of that quantity; and
- (c) if a quantity of THC or CBD that is displayed on the label does not exceed 2 mg, contain less than 75% or more than 125% of that quantity.
Variability limits — divisible cannabis products
97.1 (1) If a cannabis product that is not in discrete units is represented as being able to be divided into discrete units, each represented unit must not contain
- (a) a quantity of THC that is less than 75% or more than 125% of the quantity of THC in each of the other represented units, taking into account the potential to convert THCA into THC; and
- (b) a quantity of CBD that is less than 75% or more than 125% of the quantity of CBD in each of the other represented units, taking into account the potential to convert CBDA into CBD.
Divisible units
(2) If a cannabis product is in discrete units that are represented as being able to be divided into discrete sub-units, each represented sub-unit must not contain
- (a) a quantity of THC that is less than 75% or more than 125% of the quantity of THC in each of the other represented sub-units, taking into account the potential to convert THCA into THC; and
- (b) a quantity of CBD that is less than 75% or more than 125% of the quantity of CBD in each of the other represented sub-units, taking into account the potential to convert CBDA into CBD.
Sale
98 The following cannabis products must not be sold or distributed:
- (a) a cannabis product that is intended to be used in the area of the human eye bounded by the supraorbital and infraorbital ridges, including the eyebrows, the skin underlying the eyebrows, the eyelids, the eyelashes, the conjunctival sac of the eye, the eyeball and the soft tissue that lies below the eye and within the infraorbital ridge; and
- (b) a cannabis product that is intended to be used on damaged or broken skin or to penetrate the skin barrier other than by absorption.
24 Sections 101 and 102 of the Regulations, and the heading that precedes section 101, are replaced by the following:
Cannabis Extracts and Cannabis Topicals
Things injurious to health
101 (1) A cannabis extract, or cannabis topical, that is a cannabis product — or that is contained in a cannabis accessory that is a cannabis product — must not contain or have on it anything that may cause injury to the health of the user when the cannabis product is used as intended or in a reasonably foreseeable way.
Exception
(2) Subsection (1) does not, in respect of a cannabis extract that is intended to be combusted and inhaled, prohibit a thing that may cause injury as result of the intended combustion and inhalation.
Things that do not cause injury
(3) For the purposes of subsection (1), a cannabis extract or cannabis topical does not contain or have on it a thing that may cause injury to the health of the user by reason only that it contains or has on it
- (a) anything referred to in item 1 or 3 of Schedule 1 to the Act;
- (b) residues of a pest control product that is registered for use on cannabis under the Pest Control Products Act, or that is otherwise authorized for use under that Act, if the residues are within any maximum residue limits that are specified in relation to cannabis under section 9 or 10 of that Act; or
- (c) microbial or chemical contaminants — other than residues of a pest control product referred to in paragraph (b) — if the contaminants are within generally accepted tolerance limits for human use that are
- (i) established in a publication referred to in Schedule B to the Food and Drugs Act, and
- (ii) appropriate for the intended use and any reasonably foreseeable use of the cannabis product.
Microbial and chemical contaminants
101.1 A cannabis extract, or cannabis topical, that is a cannabis product — or that is contained in a cannabis accessory that is a cannabis product — must not contain or have on it microbial or chemical contaminants unless the contaminants are within generally accepted tolerance limits for human use that are
- (a) established in a publication referred to in Schedule B to the Food and Drugs Act; and
- (b) appropriate for the intended use and any reasonably foreseeable use of the cannabis product.
Maximum yield quantity
101.2 Subject to subsection 97(1), a cannabis extract, or cannabis topical, that is a cannabis product — or that is contained in a cannabis accessory that is a cannabis product — must not exceed a maximum yield quantity of 1000 mg of THC per immediate container, taking into account the potential to convert THCA into THC.
Cannabis extract — content
101.3 (1) A cannabis extract that is a cannabis product — or that is contained in a cannabis accessory that is a cannabis product — must not contain any ingredients other than
- (a) carrier substances;
- (b) flavouring agents; and
- (c) substances that are necessary to maintain the quality or stability of the cannabis product.
Prohibited ingredients
(2) The following ingredients must not be used to produce a cannabis extract referred to in subsection (1):
- (a) ingredients that are listed in column 1 of Schedule 2 to the Tobacco and Vaping Products Act; or
- (b) sugars or sweeteners or sweetening agents, as those terms are defined in subsection B.01.001(1) of the Food and Drug Regulations.
Permitted ingredients — inhaled extracts
(3) An ingredient — other than a flavouring agent — must not be used to produce a cannabis extract referred to in subsection (1) that is intended for inhalation unless
- (a) a standard for the ingredient is set out in a publication referred to in Schedule B to the Food and Drugs Act; and
- (b) the ingredient complies with the standard.
Ethyl alcohol — ingested extracts
(4) A cannabis extract referred to in subsection (1) must not contain ethyl alcohol unless
- (a) the cannabis extract is intended to be ingested; and
- (b) the net weight of the cannabis extract in each immediate container of the cannabis product does not exceed 7.5 g.
Uniform distribution — cannabinoids and terpenes
101.4 The cannabinoids and terpenes in a cannabis extract, or cannabis topical, that is a cannabis product — or that is contained in a cannabis accessory that is a cannabis product — must be uniformly distributed throughout the cannabis extract or cannabis topical.
Cannabis extract — external body surfaces
101.5 A cannabis extract that is a cannabis product — or that is contained in a cannabis accessory that is a cannabis product — must not be represented for use on external body surfaces, including hair and nails.
Edible Cannabis
Ingredients — edible cannabis
102 (1) Edible cannabis that is a cannabis product — or that is contained in a cannabis accessory that is a cannabis product — must not contain any ingredients other than food and food additives.
Temporarily marketed foods
(2) A food that is described in a Temporary Marketing Authorization Letter issued under subsection B.01.054(1) of the Food and Drug Regulations must not be used as an ingredient to produce edible cannabis referred to in subsection (1) and must not be a constituent of such an ingredient.
Meat products, poultry products and fish
(3) A meat product, poultry product or fish, other than a food additive, must not be used as an ingredient to produce edible cannabis referred to in subsection (1) — and must not be a constituent of such an ingredient — unless the meat product, poultry product or fish
- (a) has been produced by a person that is authorized to produce it under the laws of a province or the Safe Food for Canadians Act — or is imported in accordance with that Act; and
- (b) has a water activity that does not exceed 0.85 at the time at which the ingredient is obtained by the holder of the licence for processing that is producing the edible cannabis.
Self-produced food
(4) A holder of a licence for processing that has produced a food may use it as an ingredient to produce edible cannabis referred to in subsection (1) — or as a constituent of such an ingredient — only if
- (a) the food is not a meat product, poultry product or fish; and
- (b) the sale of the food would not be prohibited under section 4 of the Food and Drugs Act.
Food additives
(5) A holder of a licence for processing may use a food additive as an ingredient to produce edible cannabis referred to in subsection (1) only if
- (a) the edible cannabis would be a food that is the subject of a marketing authorization if the edible cannabis did not contain or have on it anything referred to in item 1 or 3 of Schedule 1 to the Act;
- (b) the marketing authorization permits the food additive to be in or on the food;
- (c) the conditions under which the marketing authorization permits the food additive to be in or on the food — including any maximum levels of use — are complied with; and
- (d) the food additive is not caffeine or caffeine citrate.
Vitamins and mineral nutrients
(6) A vitamin or mineral nutrient must not be used as an ingredient to produce edible cannabis referred to in subsection (1) unless its use is permitted under subsection (5).
Definitions
(7) The following definitions apply in this section.
fish has the same meaning as in section 1 of the Safe Food for Canadians Regulations. (poisson)
marketing authorization, except in subsection (2), has the same meaning as in subsection B.01.001(1) of the Food and Drug Regulations. (autorisation de mise en marché)
meat product has the same meaning as in subsection B.01.001(1) of the Food and Drug Regulations. (produit de viande)
mineral nutrient has the same meaning as in subsection D.02.001(1) of the Food and Drug Regulations except that it does not include sodium, potassium or chloride or compounds that include these elements. (minéral nutritif)
poultry product has the same meaning as in subsection B.01.001(1) of the Food and Drug Regulations. (produit de volaille)
vitamin has the same meaning as in subsection D.01.002(1) of the Food and Drug Regulations. (vitamine)
water activity means the ratio of the water vapour pressure of a meat product, poultry product or fish to the vapour pressure of pure water, at the same temperature and pressure. (activité de l'eau)
Prohibited things
102.1 (1) Edible cannabis that is a cannabis product — or that is contained in a cannabis accessory that is a cannabis product — must not contain or have on it a thing, or an amount of a thing, that would cause the sale of the edible cannabis to be prohibited under any of paragraphs 4(1)(a) to (d) of the Food and Drugs Act if the edible cannabis was a food to which that Act applies.
Not poisonous, harmful or adulterated
(2) Edible cannabis does not have a poisonous or harmful substance in or on it, within the meaning of paragraph 4(1)(a) of the Food and Drugs Act, and is not adulterated, within the meaning of paragraph 4(1)(d) of that Act, by reason only that it contains or has on it
- (a) anything referred to in item 1 or 3 of Schedule 1 to the Act;
- (b) residues of a pest control product that is registered for use on cannabis under the Pest Control Products Act, or is otherwise authorized for use under that Act, if the residues are within any maximum residue limits that are specified in relation to cannabis under section 9 or 10 of that Act; or
- (c) microbial or chemical contaminants — other than residues of a pest control product referred to in paragraph (b) — if the contaminants are within generally accepted tolerance limits for human use that are
- (i) established in a publication referred to in Schedule B to the Food and Drugs Act, and
- (ii) appropriate for a product that is to be ingested.
Caffeine
102.2 Edible cannabis that is a cannabis product — or that is contained in a cannabis accessory that is a cannabis product — must not contain or have on it caffeine unless
- (a) the caffeine has been introduced through the use of ingredients that naturally contain caffeine; and
- (b) the total amount of caffeine in each immediate container of the cannabis product does not exceed 30 mg.
Ethyl alcohol
102.3 Edible cannabis that is a cannabis product — or that is contained in a cannabis accessory that is a cannabis product — must not contain ethyl alcohol unless the concentration of ethyl alcohol does not exceed 0.5% w/w of the edible cannabis.
Products requiring refrigeration
102.4 It is prohibited to sell or distribute edible cannabis that is a cannabis product — or that is contained in a cannabis accessory that is a cannabis product — if the unopened immediate container must be stored at or below 4°C to prevent the cannabis product from becoming contaminated before its durable life date.
Hermetically-sealed containers
102.5 (1) It is prohibited to sell or distribute edible cannabis that is a cannabis product — or that is contained in a cannabis accessory that is a cannabis product — in a hermetically-sealed container if any component of the edible cannabis has a pH that exceeds 4.6 and a water activity that exceeds 0.85.
Definitions
(2) The following definitions apply in subsection (1).
hermetically-sealed container means a container that, due to its design, is secure against the entry of micro-organisms, including spores. (contenant hermétiquement scellé)
water activity means the ratio of the water vapour pressure of the component to the vapour pressure of pure water, at the same temperature and pressure. (activité de l'eau)
Irradiation
102.6 A holder of a licence for processing must not irradiate edible cannabis unless
- (a) the edible cannabis would be a food that is listed in column 1 of item 3 or 4 of the table to Division 26 of Part B of the Food and Drug Regulations if the edible cannabis did not contain or have on it a thing that is referred to in item 1 or 3 of Schedule 1 to the Act; and
- (b) the holder satisfies the requirements set out in paragraphs B.26.003(2)(a) and (b) and subsection B.26.004(1) of those Regulations in respect of the edible cannabis.
Multiple units
102.7 It is prohibited to sell or distribute edible cannabis that is a cannabis product — or that is contained in a cannabis accessory that is a cannabis product — if the immediate container contains multiple discrete units unless the properties of each unit, including size but excluding flavour and colour, are consistent.
Maximum yield quantity
102.8 Subject to subsection 97(2), edible cannabis that is a cannabis product — or that is contained in a cannabis accessory that is a cannabis product — must not exceed a maximum yield quantity of 10 mg of THC per immediate container, taking into account the potential to convert THCA into THC.
25 Sections 103 and 104 of the Regulations are replaced by the following:
Flavour
103 A cannabis accessory that is a cannabis product, or that is packaged with a cannabis product, must not impart a characterizing flavour to the cannabis.
Dispensing limit
103.1 Each activation of the following cannabis accessories must not dispense a quantity of cannabis extract that has a maximum yield quantity that exceeds 10 mg of THC, taking into account the potential to convert THCA into THC:
- (a) a cannabis accessory that is a cannabis product and that dispenses a cannabis extract that is intended for ingestion or nasal, rectal or vaginal use; or
- (b) a cannabis accessory that is packaged with, and that is intended to dispense, a cannabis extract that is a cannabis product and that is intended for ingestion or nasal, rectal or vaginal use.
Psychological effects, abuse liability and toxicity
104 (1) A component of a cannabis product — other than a component referred to in item 1 or 3 of Schedule 1 to the Act — and a cannabis accessory that is packaged with a cannabis product must not, through any means other than heating or combustion,
- (a) alter or enhance the psychological effects of the cannabis product in a manner that may cause injury to the health of the user when the cannabis product is used as intended or in a reasonably foreseeable way;
- (b) increase the potential for abuse liability of the cannabis product when the cannabis product is used as intended or in a reasonably foreseeable way; or
- (c) increase the toxicity of the cannabis product when the cannabis product is used as intended or in a reasonably foreseeable way.
Exceptions
(2) Subsection (1) does not prohibit the presence of
- (a) ethyl alcohol in a cannabis product referred to in subsection 101.3(4) or section 102.3 if the conditions set out in that subsection or section, as the case may be, are met; and
- (b) caffeine in or on a cannabis product referred to in section 102.2 if the conditions set out in that section are met.
26 (1) The definition immediate container in section 105 of the Regulations is repealed.
(2) Section 105 of the Regulations is renumbered as subsection 105(1) and is amended by adding the following in alphabetical order:
common name, with respect to edible cannabis, has the same meaning as in subsection B.01.001(1) of the Food and Drug Regulations. (nom usuel)
daily value means
- (a) in the case of a nutrient set out in column 1 of Part 1 of the Table of Daily Values, as defined in subsection B.01.001(1) of the Food and Drug Regulations, the quantity set out in column 3; and
- (b) in the case of a nutrient set out in column 1 of Part 2 of the table referred to in paragraph (a), the quantity set out in column 4. (valeur quotidienne)
energy value means, in respect of a cannabis product, the amount of energy made available to a person's body when the chemical components of the cannabis product, including protein, fat, carbohydrate and alcohol, are metabolized following ingestion of the cannabis product by the person. (valeur énergétique)
exterior display surface means the area on the exterior surface of an immediate container to which a label is applied and that is visible under customary conditions of purchase and use. (espace extérieur d'affichage)
fat has the same meaning as in subsection B.01.400(1) of the Food and Drug Regulations. (lipides)
food allergen has the same meaning as in subsection B.01.010.1(1) of the Food and Drug Regulations. (allergène alimentaire)
food allergen source, gluten source and added sulphites statement means a statement appearing on the label of any container in which edible cannabis — or a cannabis accessory that contains edible cannabis — that is a cannabis product is packaged that indicates the source of a food allergen or gluten that is present in the cannabis product or the presence in the product of added sulphites in a total amount of 10 p.p.m. or more. (mention des sources d'allergènes alimentaires ou de gluten et des sulfites ajoutés)
gluten has the same meaning as in subsection B.01.010.1(1) of the Food and Drug Regulations. (gluten)
INCI name has the same meaning as in subsection 2(1) of the Cosmetic Regulations. (appellation INCI)
label does not include a panel referred to in paragraph 132.26(1)(b). (étiquette)
p.p.m. means parts per million by weight. (p.p.m.)
saturated fatty acids, saturated fat, saturates or saturated has the same meaning as in subsection B.01.001(1) of the Food and Drug Regulations. (acides gras saturés, graisses saturées, gras saturés, lipides saturés ou saturés)
sugars-based ingredient has the same meaning as in subsection B.01.001(1) of the Food and Drug Regulations. (ingrédient à base de sucres)
sulphites means one or more of the following food additives:
- (a) potassium bisulphite;
- (b) potassium metabisulphite;
- (c) sodium bisulphite;
- (d) sodium dithionite;
- (e) sodium metabisulphite;
- (f) sodium sulphite;
- (g) sulphur dioxide; and
- (h) sulphurous acid. (sulfites)
trans fatty acids, trans fat or trans has the same meaning as in subsection B.01.001(1) of the Food and Drug Regulations. (acides gras trans, graisses trans, gras trans, lipides trans ou trans)
(3) Section 105 of the Regulations is amended by adding the following after subsection (1):
Interpretation — panel
(2) For the purposes of sections 112 to 117, 121 and 132.12, subsections 132.26(2) to (8) and sections 132.27 to 132.3, panel means a panel referred to in paragraph 132.26(1)(b).
27 Section 112 of the Regulations is replaced by the following:
Image
112 Except as otherwise provided under the Act, any other Act of Parliament or any provincial Act, the interior surface, exterior surface and panel of any container in which a cannabis product is packaged must not display any image.
28 (1) Subsection 113(1) of the Regulations is replaced by the following:
Uniform colour
113 (1) Except as otherwise provided under the Act, any other Act of Parliament or any provincial Act, the colour of the interior surface, exterior surface and panel of any container in which a cannabis product is packaged must be one uniform colour. However, the colour of each surface and the panel may be different.
(2) The portion of subsection 113(2) of the Regulations before paragraph (a) is replaced by the following:
Colour — other requirements
(2) The colour of the interior surface, exterior surface and panel must meet the following requirements:
(3) Subsection 113(3) of the Regulations is replaced by the following:
Exception
(3) Despite subsection (2),
- (a) an interior surface that is made of metal may be the colour of the metal; and
- (b) an exterior surface of an immediate container that is made of metal, excluding the label or any image, may be the colour of the metal.
29 Section 114 of the Regulations is repealed.
30 Subsection 115(1) of the Regulations is replaced by the following:
Texture
115 (1) Except as otherwise provided under the Act, any other Act of Parliament or any provincial Act, the interior surface, exterior surface and panel of any container in which a cannabis product is packaged and any covering of such a container must have a smooth texture without any raised features, embossing, decorative ridges, bulges or other irregularities.
31 Sections 116 and 117 of the Regulations are replaced by the following:
Hidden features
116 (1) The interior surface, exterior surface and panel of any container in which a cannabis product is packaged and any covering of such a container must not include any hidden feature that is designed to change the appearance of the container, covering or panel, such as heat-activated ink or a feature that is visible only through technological means, except a feature that is used to prevent counterfeiting.
Feature designed to change the surface area
(2) Subject to section 132.26, the interior surface and exterior surface of any container in which a cannabis product is packaged and any covering of such a container must not include any feature that is designed to change the surface area of the container or covering, such as a fold-out panel.
Scent and sound
117 The interior surface, exterior surface and panel of any container in which a cannabis product is packaged, and any covering of such a container, must not be capable of emitting a scent or sound.
32 Section 121 of the Regulations is replaced by the following:
Cut-out window
121 The interior surface, exterior surface and panel of any container in which a cannabis product is packaged must not include any cut-out window.
33 The Regulations are amended by adding the following after section 122:
Wrapper
122.1 A wrapper must not be used with respect to a cannabis product unless
- (a) it is only in direct contact with
- (i) a cannabis extract, a cannabis topical or edible cannabis, and
- (ii) the immediate container of the cannabis product; and
- (b) it is required to maintain the quality or stability of the cannabis product.
Packaging requirements — other Regulations
122.11 The immediate container in which edible cannabis is packaged and any wrapper must meet the requirements set out in Division 23 of Part B of the Food and Drug Regulations and subparagraphs 186(a)(i), (ii) and (v) to (vii) of the Safe Food for Canadians Regulations as if the cannabis was a food for the purposes of that Division and those provisions.
Maximum quantity — cannabis extract
122.12 The immediate container of a cannabis extract that is a cannabis product must not contain more than 90 mL of extract that is in non-solid form at a temperature of 22 ± 2°C.
Outermost container
122.13 The outermost container in which a cannabis product is packaged and to which a label that meets the requirements of these Regulations is applied must not contain
- (a) food;
- (b) more than one class of cannabis set out in Schedule 4 to the Act; or
- (c) more than one immediate container.
Pressurized containers
122.14 The immediate container of a cannabis product — other than edible cannabis that is a cannabis product and that is in liquid form at a temperature of 22 ± 2°C — must not be a pressurized container.
Dispensing controls for cannabis extracts
122.15 The immediate container of a cannabis extract that is a cannabis product and that is not in discrete units must
- (a) not permit the extract to be easily poured or drunk directly from the container; and
- (b) contain an integrated dispensing mechanism that dispenses no more than 10 mg of THC per activation, taking into account the potential to convert THCA into THC, if the cannabis extract
- (i) is in a liquid form at a temperature of 22 ± 2°C,
- (ii) is not intended only for inhalation, and
- (iii) contains at least 10 mg of THC, taking into account the potential to convert THCA into THC.
34 (1) The portion of subparagraph 123(1)(c)(v) of the Regulations before clause (A) is replaced by the following:
- (v) except in the case of a cannabis plant, cannabis plant seeds or edible cannabis, either
(2) Paragraph 123(1)(f) of the Regulations is replaced by the following:
- (f) in the case of a cannabis product that contains THC in a concentration greater than 10 μg/g, taking into account the potential to convert THCA into THC, the standardized cannabis symbol that must be obtained from the Minister in the form of an electronic file.
(3) Paragraph 123(2)(b) of the Regulations is replaced by the following:
- (b) its microbial and chemical contaminants remain within the limits referred to in
- (i) in the case of dried or fresh cannabis, subsection 93(3), or
- (ii) in the case of a cannabis extract or a cannabis topical, paragraph 101(3)(c).
35 The Regulations are amended by adding the following after section 123:
Wrapper
123.1 (1) The interior and exterior surface of a wrapper must
- (a) not display any brand element;
- (b) not display any image or information;
- (c) be one uniform colour but the colour of each surface may be different;
- (d) not be fluorescent, have fluorescent properties in the ink or have pigments that absorb ultraviolet energy and transmit it as a longer wavelength, such as the Pantone 800 series;
- (e) have a smooth texture without any embossing, or decorative ridges;
- (f) not include any hidden feature that is designed to change the appearance of the wrapper, such as heat-activated ink or a feature that is visible only through technological means; and
- (g) not be capable of emitting a scent or sound.
Standardized cannabis symbol
(2) Despite paragraph (1)(b), the standardized cannabis symbol that must be obtained from the Minister in the form of an electronic file must be clearly and prominently displayed on the exterior surface of any wrapper that is in direct contact with a cannabis extract, a cannabis topical or edible cannabis that contains THC in a concentration greater than 10 μg/g, taking into account the potential to convert THCA into THC.
Requirements
(3) The standardized cannabis symbol must meet the following conditions:
- (a) it must be at least 1.27 cm by 1.27 cm in size;
- (b) it must be displayed with a white border of at least 2 points on all sides; and
- (c) if a change is made to the size of the symbol, its dimensions must be proportional vertically and horizontally.
36 Sections 126 and 127 of the Regulations are repealed.
37 (1) Subsection 130(1) of the Regulations is replaced by the following:
Presentation of information — general requirement
130 (1) All information that is included on a label must be in English and in French, except for the INCI name and the EU trivial name.
(2) The portion of paragraph 130(3)(e) of the Regulations before subparagraph (i) is replaced by the following:
- (e) in the case of the information required under paragraphs 124(d) to (g), 125(b) to (e), 132.1(d) to (g), 132.11(b) to (e), 132.14(d) to (g), 132.15(b) to (e), 132.17(1)(c) to (j) and 132.18(1)(b) to (e), it must be
(3) Section 130 of the Regulations is amended by adding the following after subsection (10):
Location of information on irradiation — edible cannabis
(11) Information that is required to be included on a label under paragraph 132.17(1)(p) or 132.18(1)(k) must be displayed on the principal display panel, or if there are separate principal display panels for English and French, on each principal display panel.
38 The Regulations are amended by adding the following after section 132:
Cannabis extract — discrete unit
132.1 In the case of a cannabis extract — or a cannabis accessory that contains cannabis extract — that is in discrete units, the label of any container in which the cannabis product is packaged must also include the following information:
- (a) in the case where the cannabis extract is in solid form, the net weight, in grams, and in any other case, the net volume, in millilitres, of the cannabis extract;
- (b) the number of units;
- (c) in the case where the cannabis extract is in solid form, the net weight, in grams of each unit, and in any other case, the net volume, in millilitres, of each unit;
- (d) the quantity of THC, in milligrams, in each unit preceded by "THC per unit";
- (e) the quantity of THC, in milligrams, that each unit could yield, taking into account the potential to convert THCA into THC, preceded by "Total THC per unit";
- (f) the quantity of CBD, in milligrams, in each unit, preceded by "CBD per unit";
- (g) the quantity of CBD, in milligrams, that each unit could yield, taking into account the potential to convert CBDA into CBD, preceded by "Total CBD per unit";
- (h) a list of the ingredients of the cannabis extract;
- (i) the name of any food allergen that is present in the cannabis extract, except as a result of cross-contamination;
- (j) the identity of the cannabis product in terms of its common name or in terms of its function; and
- (k) the intended use of the cannabis product.
Cannabis extract — not in discrete units
132.11 In the case of a cannabis extract — or a cannabis accessory that contains cannabis extract — that is not in discrete units, the label of any container in which the cannabis product is packaged must also include the following information:
- (a) in the case where the cannabis extract is in solid form, the net weight, in grams, and in any other case, the net volume, in millilitres, of the cannabis extract;
- (b) either the concentration of THC, in milligrams per millilitre, or the percentage of THC w/w in the cannabis extract preceded by "THC";
- (c) either the concentration of THC, in milligrams per millilitre, or the percentage of THC w/w that the cannabis extract could yield, taking into account the potential to convert THCA into THC, preceded by "Total THC";
- (d) either the concentration of CBD, in milligrams per millilitre, or the percentage of CBD w/w in the cannabis extract preceded by "CBD";
- (e) either the concentration of CBD, in milligrams per millilitre, or the percentage of CBD w/w that the cannabis extract could yield, taking into account the potential to convert CBDA into CBD, preceded by "Total CBD";
- (f) a list of the ingredients of the cannabis extract;
- (g) the name of any food allergen that is present in the cannabis extract, except as a result of cross-contamination;
- (h) the identity of the cannabis product in terms of its common name or in terms of its function; and
- (i) the intended use of the cannabis product.
Flavours
132.12 It is prohibited to display on a cannabis extract — or on a cannabis accessory that contains cannabis extract — or on its package, label or panel an indication or illustration, including a brand element, that could cause a person to believe that the cannabis extract or the cannabis accessory, as the case may be, has a flavour set out in column 1 of Schedule 3 to the Tobacco and Vaping Products Act, other than the flavour cannabis.
List of ingredients — cannabis extract
132.13 (1) The list of ingredients of a cannabis extract — or of a cannabis accessory that contains cannabis extract — must
- (a) be preceded by the term "Ingredients" in the English version and the term "Ingrédients" in the French version;
- (b) be shown without any intervening printed, written or graphic material appearing between the term referred to in paragraph (a) and the first ingredient in the list; and
- (c) list the ingredients
- (i) in descending order of predominance, in their concentration by weight or volume, and
- (ii) only by their common name or chemical name.
Ingredients in concentration of 1% or less
(2) Despite subparagraph (1)(c)(i), ingredients that are present at a concentration of 1% or less may be listed in any order after the ingredients that are present at a concentration of more than 1%.
Cannabis topical — discrete unit
132.14 In the case of a cannabis topical — or a cannabis accessory that contains a cannabis topical — that is in discrete units, the label of any container in which the cannabis product is packaged must also include the following information:
- (a) in the case where the cannabis topical is in solid form, the net weight, in grams, and in any other case, the net volume, in millilitres, of the cannabis topical;
- (b) the number of units;
- (c) in the case where the cannabis topical is in solid form, the net weight, in grams of each unit, and in any other case, the net volume, in millilitres, of each unit;
- (d) either the quantity, in milligrams, or the concentration, in milligrams per millilitre, of THC in each unit preceded by "THC per unit";
- (e) either the quantity, in milligrams, or the concentration, in milligrams per millilitre, of THC that each unit could yield, taking into account the potential to convert THCA into THC, preceded by "Total THC per unit";
- (f) either the quantity, in milligrams, or the concentration, in milligrams per millilitre, of CBD in each unit, preceded by "CBD per unit";
- (g) either the quantity, in milligrams, or the concentration, in milligrams per millilitre, of CBD that each unit could yield, taking into account the potential to convert CBDA into CBD, preceded by "Total CBD per unit";
- (h) a list of the ingredients of the cannabis topical;
- (i) the identity of the cannabis product in terms of its common name or in terms of its function;
- (j) the intended use of the cannabis product;
- (k) the warning "Do not swallow or apply internally or to broken, irritated or itching skin / Ne pas avaler ou appliquer sur une surface interne ou sur une surface éraflée, irritée ou en proie à la démangeaison"; and
- (l) the directions for use of the cannabis product.
Cannabis topical — not in discrete units
132.15 In the case of a cannabis topical — or a cannabis accessory that contains cannabis topical — that is not in discrete units, the label of any container in which the cannabis product is packaged must also include the following information:
- (a) in the case where the cannabis topical is in solid form, the net weight, in grams, and in any other case, the net volume, in millilitres, of the cannabis topical;
- (b) either the quantity, in milligrams, or the concentration, in milligrams per millilitre, of THC in the cannabis topical preceded by "THC";
- (c) either the quantity, in milligrams, or the concentration, in milligrams per millilitre, of THC that the cannabis topical could yield, taking into account the potential to convert THCA into THC, preceded by "Total THC";
- (d) either the quantity, in milligrams, or the concentration, in milligrams per millilitre, of CBD in the cannabis topical preceded by "CBD";
- (e) either the quantity, in milligrams, or the concentration, in milligrams per millilitre, of CBD that the cannabis topical could yield, taking into account the potential to convert CBDA into CBD, preceded by "Total CBD";
- (f) a list of the ingredients of the cannabis topical;
- (g) the identity of the cannabis product in terms of its common name or in terms of its function;
- (h) the intended use of the cannabis product;
- (i) the warning "Do not swallow or apply internally or to broken, irritated or itching skin / Ne pas avaler ou appliquer sur une surface interne ou sur une surface éraflée, irritée ou en proie à la démangeaison"; and
- (j) the directions for use of the cannabis product.
List of ingredients – cannabis topical
132.16 (1) The list of ingredients of a cannabis topical —or of a cannabis accessory that contains a cannabis topical — must
- (a) be preceded by the term "Ingredients" in the English version and the term "Ingrédients" in the French version;
- (b) be shown without any intervening printed, written or graphic material appearing between the term referred to in paragraph (a) and the first ingredient in the list; and
- (c) list the ingredients in descending order of predominance, in their concentration by weight or volume, as follows:
- (i) by their INCI name only,
- (ii) in the case where an ingredient has no INCI name, by its chemical name,
- (iii) in the case of a botanical, by specifying at least the genus and species portions of its INCI name or, if it has no INCI name, by its chemical name, or
- (iv) in the case where an ingredient is included in the schedule to the Cosmetic Regulations, by its EU trivial name set out in column 1 of the schedule or by the appropriate English and French equivalents set out in columns 2 and 3.
Ingredients in concentration of 1% or less
(2) Despite paragraph (1)(c), ingredients that are present at a concentration of 1% or less and all colouring agents, regardless of their concentration, may be listed in any order after the ingredients that are present at a concentration of more than 1%.
Fragrance
(3) The word "parfum" may be inserted at the end of the list of ingredients to indicate that an ingredient has been added to the cannabis topical to produce a particular fragrance.
Interpretation — botanical
(4) For the purposes of this section, botanical means an ingredient that is directly derived from a plant and that has not been chemically modified before it is used in the production of a cannabis topical.
Edible cannabis — discrete unit
132.17 (1) In the case of edible cannabis — or a cannabis accessory that contains edible cannabis — that is in discrete units, the label of any container in which the cannabis product is packaged must also include the following information:
- (a) in the case where the edible cannabis is in solid form, the net weight, in grams, and in any other case, the net volume, in millilitres, of the edible cannabis;
- (b) the number of units;
- (c) the quantity of THC, in milligrams, in each unit preceded by "THC per unit";
- (d) the quantity of THC, in milligrams, that each unit could yield, taking into account the potential to convert THCA into THC, preceded by "Total THC per unit";
- (e) the quantity of THC, in milligrams, in the edible cannabis preceded by "THC";
- (f) the quantity of THC, in milligrams, that the edible cannabis could yield, taking into account the potential to convert THCA into THC, preceded by "Total THC";
- (g) the quantity of CBD, in milligrams, in each unit, preceded by "CBD per unit";
- (h) the quantity of CBD, in milligrams, that each unit could yield, taking into account the potential to convert CBDA into CBD, preceded by "Total CBD per unit";
- (i) the quantity of CBD, in milligrams, in the edible cannabis preceded by "CBD";
- (j) the quantity of CBD, in milligrams, that the edible cannabis could yield, taking into account the potential to convert CBDA into CBD, preceded by "Total CBD";
- (k) a list of the ingredients of the edible cannabis, including constituents, if any;
- (l) the source of any food allergen or gluten present in the edible cannabis, except as a result of cross-contamination,
- (i) in a food allergen source, gluten source and added sulphites statement, if the food allergen or gluten
- (A) is, or is present in, an ingredient that is not shown in the list of ingredients, but is not a constituent of that ingredient or present in a constituent of that ingredient, or
- (B) is, or is present in, a constituent and neither the constituent nor the ingredient in which it is present is shown in the list of ingredients, or
- (ii) in all other cases, either in the list of ingredients or in a food allergen source, gluten source and added sulphites statement;
- (i) in a food allergen source, gluten source and added sulphites statement, if the food allergen or gluten
- (m) if the total amount of sulphites that are present in the edible cannabis is 10 p.p.m. or more, the sulphites must be shown,
- (i) in the case where at least one sulphite is required to be shown in the list of ingredients pursuant to these Regulations, in the list of ingredients, and they may also be shown in a food allergen source, gluten source and added sulphites statement, or
- (ii) in any other case, in the list of ingredients, in a food allergen source, gluten source and added sulphites statement or in both;
- (n) a nutrition facts table that contains only the information set out in column 1 of the table to section 132.21, expressed using a description set out in column 2, in the unit set out in column 3 and in the manner set out in column 4;
- (o) the common name of the cannabis product;
- (p) if the edible cannabis is irradiated under section 102.6, the symbol described in subsection B.01.035(5) of the Food and Drug Regulations and one of the following statements or a statement that has the same meaning:
- (i) "treated with radiation",
- (ii) "treated by irradiation", or
- (iii) "irradiated"; and
- (q) if an irradiated food referred to in column 1 of the table to Division 26 of Part B of the Food and Drug Regulations is an ingredient or constituent of the edible cannabis and constitutes 10% or more of the edible cannabis, the statement "irradiated" preceding any mention of the ingredient or constituent on the label.
Ingredient not required to be listed
(2) Despite paragraph (1)(k), if one or more constituents of an ingredient are required by these Regulations to be listed in a list of ingredients, the ingredient is not required to be listed if all constituents of the ingredient are shown in the list by their common names and in accordance with subparagraph 132.2(1)(c)(i).
Risk of cross-contamination
(3) Despite paragraph (1)(l), the source of a food allergen or gluten must be shown if the label includes a declaration alerting consumers that, due to a risk of cross-contamination, the edible cannabis may contain the source of a food allergen or gluten.
Edibles containing cannabis — not in a discrete unit
132.18 (1) In the case of edible cannabis — or a cannabis accessory that contains edible cannabis — that is not in discrete units, the label of any container in which the cannabis product is packaged must also include the following information:
- (a) in the case where the edible cannabis is in solid form, the net weight, in grams, and in any other case, the net volume, in millilitres, of the edible cannabis;
- (b) the quantity of THC, in milligrams, in the edible cannabis preceded by "THC";
- (c) the quantity of THC, in milligrams, that the edible cannabis could yield, taking into account the potential to convert THCA into THC, preceded by "Total THC";
- (d) the quantity of CBD, in milligrams, in the edible cannabis preceded by "CBD";
- (e) the quantity of CBD, in milligrams, that the edible cannabis could yield, taking into account the potential to convert CBDA into CBD, preceded by "Total CBD";
- (f) a list of the ingredients of the edible cannabis, including constituents, if any;
- (g) the source of any food allergen or gluten present in the edible cannabis, except as a result of cross-contamination,
- (i) in a food allergen source, gluten source and added sulphites statement, if the food allergen or gluten
- (A) is, or is present in, an ingredient that is not shown in the list of ingredients, but is not a constituent of that ingredient or present in a constituent of that ingredient, or
- (B) is, or is present in, a constituent and neither the constituent nor the ingredient in which it is present is shown in the list of ingredients, or
- (ii) in all other cases, either in the list of ingredients or in a food allergen source, gluten source and added sulphites statement;
- (i) in a food allergen source, gluten source and added sulphites statement, if the food allergen or gluten
- (h) if the total amount of sulphites that are present in the edible cannabis is 10 p.p.m. or more, the sulphites must be shown,
- (i) in the case where at least one sulphite is required to be shown in the list of ingredients pursuant to these Regulations, in the list of ingredients, and they may also be shown in a food allergen source, gluten source and added sulphites statement, or
- (ii) in any other case, in the list of ingredients, in a food allergen source, gluten source and added sulphites statement or in both;
- (i) a nutrition facts table that contains only the information set out in column 1 of the table to section 132.21, expressed using a description set out in column 2, in the unit set out in column 3 and in the manner set out in column 4;
- (j) the common name of the cannabis product;
- (k) if the edible cannabis is irradiated under section 102.6, the symbol described in subsection B.01.035(5) of the Food and Drug Regulations and one of the following statements or a statement that has the same meaning:
- (i) "treated with radiation",
- (ii) "treated by irradiation", or
- (iii) "irradiated"; and
- (l) if an irradiated food referred to in column 1 of the table to Division 26 of Part B of the Food and Drug Regulations is an ingredient or constituent of the edible cannabis and constitutes 10% or more of the edible cannabis, the statement "irradiated" preceding any mention of the ingredient or constituent on the label.
Ingredient not required to be listed
(2) Despite paragraph (1)(f), if one or more constituents of an ingredient are required by these Regulations to be listed in a list of ingredients, the ingredient is not required to be listed if all constituents of the ingredient are shown in the list by their common names and in accordance with subparagraph 132.2(1)(c)(i).
Risk of cross-contamination
(3) Despite paragraph (1)(g), the source of a food allergen or gluten must be shown if the label of the edible cannabis includes a declaration alerting consumers that, due to a risk of cross-contamination, the edible cannabis may contain the source of a food allergen or gluten.
Durable life date required
132.19 (1) In the case of edible cannabis having a durable life of 90 days or less, the durable life date must be shown on the label of any container in which the edible cannabis is packaged.
Application of Food and Drug Regulations
(2) Any durable life date on the label of any container in which edible cannabis is packaged must be shown in accordance with subsections B.01.007(4) and (5) of the Food and Drug Regulations.
List of ingredients – edible cannabis
132.2 (1) The list of ingredients for edible cannabis — or for a cannabis accessory that contains edible cannabis — must
- (a) be preceded by the term "Ingredients" in the English version and the term "Ingrédients" in the French version;
- (b) be shown without any intervening printed, written or graphic material appearing between the term referred to in paragraph (a) and the first ingredient in the list;
- (c) list the ingredients and constituents
- (i) in descending order of their proportion of the edible cannabis or as a percentage of the edible cannabis, and the order or percentage must be the order or percentage of the ingredients or the constituents before they are combined to form the edible cannabis, and
- (ii) by the applicable name set out in column II of the table to paragraph B.01.010(3)(a) of the Food and Drug Regulations or, if none apply, by their common name only;
- (d) the constituents of an ingredient must be shown
- (i) in parentheses, immediately after the ingredient, unless the source of a food allergen or gluten is listed immediately after the ingredient, in which case the constituent of the ingredient must be listed immediately after that source,
- (ii) in descending order of their proportion of the ingredient determined before the constituents are combined to form the ingredient, and
- (iii) separated by a comma;
- (e) show the source of a food allergen or gluten
- (i) in parentheses,
- (ii) immediately after the ingredient that is shown in that list, if the food allergen or gluten
- (A) is that ingredient,
- (B) is present in that ingredient, but is not a constituent of or present in a constituent of that ingredient, or
- (C) is, or is present in, a constituent of that ingredient and the constituent is not shown in the list of ingredients,
- (iii) immediately after the constituent that is shown in the list of ingredients, if the food allergen or gluten is that constituent or is present in that constituent, and
- (iv) separated by a comma from any other source of a food allergen or gluten that is shown for the same ingredient or constituent;
- (f) show sulphites
- (i) by one of the common names "sulfites", "sulfiting agents", "sulphites" or "sulphiting agents" or individually by the applicable name set out in column I of item 21 of the table to paragraph B.01.010(3)(b) of the Food and Drug Regulations,
- (ii) in the case of the name "sodium dithionite", "sulphur dioxide" or "sulphurous acid", followed by one of the common names "sulfites", "sulfiting agents", "sulphites" or "sulphiting agents" in parentheses, unless
- (A) the term "sulfite" or "sulphite" appears in the common name of another sulphite in the list of ingredients,
- (B) one of the common names "sulfites", "sulfiting agents", "sulphites" or "sulphiting agents" is shown in parentheses following another sulphite in the list of ingredients, or
- (C) one of the common names "sulfites", "sulfiting agents", "sulphites" or "sulphiting agents" is shown in a food allergen source, gluten source and added sulphites statement on the label, and
- (iii) at the end of the list of ingredients where they may be shown in any order with the other ingredients that are shown at the end of that list in accordance with subsection (2) or in parentheses immediately after the ingredient of which they are a constituent; and
- (g) if the edible cannabis contains one or more sugars-based ingredients, show
- (i) the term "Sugars" in the English version of the list and "Sucres" in the French version of the list
- (A) despite subparagraph (c)(i), in descending order of the proportion of all the sugars-based ingredients in the edible cannabis or as a percentage of the edible cannabis, and the order or percentage must be the order or percentage of all the sugars-based ingredients before they are combined to form the product, and
- (B) separated from other ingredients by a comma, and
- (ii) each sugars-based ingredient
- (A) in descending order of its proportion of the edible cannabis as prescribed by subsection B.01.008.2(3) of the Food and Drug Regulations,
- (B) in parentheses, immediately following the term "Sugars" in the English version of the list and "Sucres" in the French version of the list, and
- (C) separated by a comma.
- (i) the term "Sugars" in the English version of the list and "Sucres" in the French version of the list
Exception — ingredients and constituents shown collectively
(2) Despite paragraph (1)(c), the ingredients and the constituents set out in column I of an item of the table to paragraph B.01.010(3)(b) of the Food and Drug Regulations may be shown collectively in the list of ingredients by the common name set out in column II of that item, except in the case where one of the ingredients or constituents referred to in that table is shown separately in the list of ingredients by its common name.
Exception — ingredients at the end of the list
(3) Despite subparagraph (1)(c)(i), the ingredients referred to in subsection B.01.008.2(4) of the Food and Drug Regulations, regardless of their proportion or percentage, may be listed at the end of the list of ingredients, in any order.
Exception — source of food allergen or gluten
(4) Despite paragraph (1)(e), the source of the food allergen or gluten is not required to be shown in parentheses immediately after the ingredient or constituent, as the case may be, if the source of the food allergen or gluten appears
- (a) in the list of ingredients
- (i) as part of the common name of the ingredient or constituent, or
- (ii) in parentheses, in accordance with subparagraph (1)(e)(i), immediately after another ingredient or constituent; or
- (b) in the food allergen source, gluten source and added sulphites statement.
Nutrition facts table
132.21 (1) The percentage of the daily value for a nutrient shown in the nutrition facts table on the label of any container in which edible cannabis is packaged must be established on the basis of the amount, by weight, of the nutrient per immediate container of edible cannabis, rounded off in the applicable manner set out in column 4 of the table to this section.
Not a significant source of a nutrient
(2) Information with respect to a nutrient set out in column 1 of the table to this section that may be expressed as "0" in the nutrition facts table may be omitted from the table if the nutrition facts table includes the statement "Not a significant source of (naming each nutrient that is omitted from the nutrition facts table in accordance with this subsection)".
Presentation
(3) Despite section 130, the nutrition facts table must be presented in accordance with the applicable format set out in the document entitled Directory of Nutrition Facts Table Format for Edible Cannabis, as amended from time to time and published by the Government of Canada on its website, having regard to matters such as order of presentation, dimensions, spacing and the use of upper and lower case letters and bold type.
TABLE
| Item | Column 1 Information |
Column 2 Description |
Column 3 Unit |
Column 4 Manner of expression |
|---|---|---|---|---|
| 1 | Immediate container size | "Per container (naming the amount of edible cannabis in the immediate container)" | The size is expressed per immediate container in grams or millilitres. | (1) The size when expressed in a metric unit is rounded off
|
| 2 | Energy value | "Calories", "Total Calories" or "Calories, Total" | The value is expressed in Calories per immediate container. | The value is rounded off
|
| 3 | Amount of fat | "Fat", "Total Fat" or "Fat, Total" | The amount is expressed
|
(1) The amount is rounded off
|
| 4 | Amount of saturated fatty acids | "Saturated Fat", "Saturated Fatty Acids", "Saturated" or "Saturates" | The amount is expressed in grams per immediate container. | The amount is rounded off
|
| 5 | Amount of trans fatty acids | "Trans Fat", "Trans Fatty Acids" or "Trans" | The amount is expressed in grams per immediate container. | The amount is rounded off
|
| 6 | The sum of saturated fatty acids and trans fatty acids | "Saturated Fat + Trans Fat", "Saturated Fatty Acids + Trans Fatty Acids", "Saturated + Trans" or "Saturates + Trans" | The sum is expressed as a percentage of the daily value per immediate container. | The percentage is rounded off
|
| 7 | Amount of cholesterol | "Cholesterol" | The amount is expressed in milligrams per immediate container. | The amount is rounded off to the nearest multiple of 5 mg. |
| 8 | Amount of sodium | "Sodium" | The amount is expressed
|
(1) The amount is rounded off
|
| 9 | Amount of carbohydrate | "Carbohydrate", "Total Carbohydrate" or "Carbohydrate, Total" | The amount is expressed in grams per immediate container. | The amount is rounded off
|
| 10 | Amount of fibre | "Fibre", "Fiber", "Dietary Fibre" or "Dietary Fiber" | The amount is expressed
|
(1) The amount is rounded off
|
| 11 | Amount of sugars | "Sugars" | The amount is expressed
|
(1) The amount is rounded off
|
| 12 | Amount of protein | "Protein" | The amount is expressed in grams per immediate container. | The amount is rounded off
|
| 13 | Amount of potassium | "Potassium" | The amount is expressed
|
(1) The amount is rounded off
|
| 14 | Amount of calcium | "Calcium" | The amount is expressed
|
(1) The amount is rounded off
|
| 15 | Amount of iron | "Iron" | The amount is expressed
|
(1) The amount is rounded off
|
Presentation of source of food allergen
132.22 (1) The source of a food allergen required to be shown under paragraph 132.17(1)(l) or 132.18(1)(g) must be shown
- (a) for a food allergen from a food referred to in one of paragraphs (a), (b) and (e) of the definition food allergen in subsection B.01.010.1(1) of the Food and Drug Regulations or derived from that food, by the name of the food as shown in the applicable paragraph, expressed in the singular or plural;
- (b) for a food allergen from the food referred to in paragraph (c) of the definition food allergen in subsection B.01.010.1(1) of the Food and Drug Regulations or derived from that food, by the name "sesame", "sesame seed" or "sesame seeds";
- (c) for a food allergen from a food referred to in one of paragraphs (d) and (f) of the definition food allergen in subsection B.01.010.1(1) of the Food and Drug Regulations or derived from that food, by the name of the food as shown in the applicable paragraph;
- (d) for a food allergen from the food referred to in paragraph (g) of the definition food allergen in subsection B.01.010.1(1) of the Food and Drug Regulations or derived from that food, by the name "soy", "soya", "soybean" or "soybeans";
- (e) for a food allergen from a food referred to in one of paragraphs (h) to (j) of the definition food allergen in subsection B.01.010.1(1) of the Food and Drug Regulations or derived from that food, by the common name of the food referred to in column II of item 6, 23 or 24 of the table to paragraph B.01.010(3)(a) of the Food and Drug Regulations, whichever is applicable; and
- (f) for a food allergen from the food referred to in paragraph (k) of the definition food allergen in subsection B.01.010.1(1) of the Food and Drug Regulations or derived from that food, by the name "mustard", "mustard seed" or "mustard seeds".
Presentation of source of gluten
(2) The source of gluten required to be shown under paragraph 132.17(1)(l) or 132.18(1)(g) must be shown
- (a) for gluten from the grain of a cereal referred to in one of subparagraphs (a)(i) to (v) of the definition gluten in subsection B.01.010.1(1) of the Food and Drug Regulations or derived from that grain, by the name of the cereal as shown in the applicable subparagraph; and
- (b) for gluten from the grain of a hybridized strain created from one or more of the cereals referred to in subparagraphs (a)(i) to (v) of the definition gluten in subsection B.01.010.1(1) of the Food and Drug Regulations or derived from that grain, by the names of the cereals as shown in the applicable subparagraphs.
Declaration on risk of cross-contamination
132.23 If the label of the container in which edible cannabis is packaged includes a declaration alerting consumers that, due to a risk of cross-contamination, the edible cannabis may contain the source of a food allergen or gluten, the declaration must
- (a) be shown immediately after the food allergen source, gluten source and added sulphites statement or, if there is none, after the list of ingredients, and must appear on the same continuous surface as the statement, if any, and the list of ingredients; and
- (b) appear without any intervening printed, written or graphic material between it and the list of ingredients or statement that immediately precedes it.
Presentation of food allergen statement
132.24 (1) A food allergen source, gluten source and added sulphites statement must
- (a) be preceded by the term "Contains" in the English version and the term "Contient" in the French version;
- (b) be shown without any intervening printed, written or graphic material appearing between the term referred to in paragraph (a) and the rest of the statement;
- (c) appear on the same continuous surface as the list of ingredients; and
- (d) include, even if any of the following information is also shown in the list of ingredients,
- (i) the source for each food allergen that is present in the edible cannabis,
- (ii) each source for the gluten that is present in the edible cannabis, and
- (iii) one of the common names "sulfites", "sulfiting agents", "sulphites" or "sulphiting agents", if the total amount of sulphites present in the edible cannabis is 10 p.p.m. or more.
No duplication
(2) Despite paragraph (1)(d), the following information is not required to be shown in the statement more than once:
- (a) the same source of a food allergen;
- (b) the same source of gluten; and
- (c) one of the common names "sulfites", "sulfiting agents", "sulphites" or "sulphiting agents".
Presentation of constituents
132.25 (1) Constituents of ingredients or of classes of ingredients set out in the table to subsection B.01.009(1) of the Food and Drug Regulations are not required to be shown on a label of the container in which edible cannabis — or a cannabis accessory that contains edible cannabis — that is a cannabis product is packaged.
Preparation or mixture
(2) Subject to subsection (3), where a preparation or mixture set out in the table to subsection B.01.009(2) of the Food and Drug Regulations is used to produce edible cannabis, the ingredients and constituents of the preparation or mixture are not required to be shown on the label of the container in which edible cannabis — or a cannabis accessory that contains edible cannabis — that is a cannabis product is packaged.
Common name
(3) Where a preparation or mixture set out in the table to subsection B.01.009(2) of the Food and Drug Regulations is used to produce edible cannabis, and the preparation or mixture has one or more of the ingredients or constituents listed in subsection B.01.009(3) of the Food and Drug Regulations, those ingredients or constituents must be shown by their common names in the list of the ingredients of the edible cannabis to which they are added as if they were ingredients of that edible cannabis.
Presentation of constituents
(4) Despite subsections (1) and (2), where any of the constituents listed in subsection B.01.009(4) of the Food and Drug Regulations is contained in an ingredient of edible cannabis set out in a table referred to in subsection (1) or (2), that constituent must be shown in the list of ingredients.
Small immediate container
132.26 (1) In the case of a cannabis product whose immediate container is too small to display the required information in accordance with these Regulations,
- (a) the label may extend beyond the exterior display surface; or
- (b) either a peel back or accordion panel may be affixed to the container.
Label or panel not easily removed
(2) The label referred to in paragraph (1)(a) and the panel must be affixed in a manner that it cannot be easily removed from the immediate container.
Panel
(3) The panel must
- (a) be able to be re-sealed;
- (b) withstand repeated openings and closings without detaching from the immediate container under customary conditions of use; and
- (c) include only the following information that cannot be included on the label because the immediate container of the product is too small to display the required information in accordance with these Regulations:
- (i) the class of cannabis set out in Schedule 4 to the Act to which the cannabis that is in the immediate container belongs,
- (ii) the recommended storage conditions,
- (iii) the packaging date,
- (iv) except in the case of a cannabis plant, cannabis plant seeds or edible cannabis, either
- (A) the expiry date in accordance with subsection 123(2), or
- (B) a statement that no expiry date has been determined,
- (v) the list of ingredients of the cannabis product, including constituents, if any,
- (vi) in the case of dried or fresh cannabis, its net weight,
- (vii) in the case of a cannabis extract,
- (A) the net weight or volume, including the net weight or volume of each unit, if the cannabis extract is in discrete units, and
- (B) the name of any food allergen that is contained in the product,
- (viii) in the case of a cannabis topical,
- (A) the net weight or volume, including the net weight or volume of each unit, if the cannabis topical is in discrete units, and
- (B) the directions for use of the product, and
- (ix) in the case of edible cannabis,
- (A) the net weight or volume,
- (B) the durable life date,
- (C) the source of any food allergen or gluten present in the edible cannabis, except as a result of cross-contamination,
- (D) sulphites that are present in the edible cannabis in an amount of 10 p.p.m. or more, and
- (E) the nutrition facts table.
Interpretation — information on panel
(4) The information included on the panel must be shown in accordance with the provisions of these Regulations with respect to a label as if the panel is a label under those provisions.
Brand element
(5) The panel must not display any brand element.
Statement on location of information
(6) The label of an immediate container in which a cannabis product is packaged and to which a panel is affixed must include a statement that clearly indicates the location of any information required under these Regulations that is not included on the label.
Image
(7) The label referred to in subsection (6) may include an image that is printed in black and white and that provides instructions on how to open the panel.
Information on exterior display surface
(8) The label referred to in subsection (6) must only include information that is required under these Regulations but may also include
- (a) a bar code, in accordance with section 122;
- (b) a brand element, in accordance with subsection (9); and
- (c) an image, in accordance with subsection 130(10).
Exception — brand element
(9) Despite paragraphs 130(9)(d) and (e), a brand element included on a label that is extended beyond the exterior display surface or on a label of a container to which a panel is affixed must
- (a) if the brand element is an image, be 1.27 cm by 1.27 cm in size or smaller; or
- (b) if the brand element is text only, its type size must be 7 points or smaller.
Prohibited representations — health and cosmetic benefits
132.27 It is prohibited to make an express or implied representation, including by way of a brand element, on a cannabis product — or on the package, label or panel of a cannabis product — if there are reasonable grounds to believe that the representation could create the impression that the cannabis product has health or cosmetic benefits.
Prohibited representation — energy value and amount of nutrient
132.28 (1) It is prohibited to make an express or implied representation, including by way of a brand element, on edible cannabis that is a cannabis product — or on the package, label or panel of such a cannabis product — with respect to energy value or the amount of a nutrient with respect to items 2 to 15 of the table to section 132.21 and items 5 to 37 of the table to section B.01.402 of the Food and Drug Regulations.
Exception — nutrition facts table
(2) For greater certainty, subsection (1) does not limit the application of paragraphs 132.17(1)(n) or 132.18(1)(i).
Special diets
132.29 It is prohibited to make an express or implied representation, including by way of a brand element, on edible cannabis that is a cannabis product — or on the package, label or panel of such a cannabis product — if there are reasonable grounds to believe that the representation could create the impression that the cannabis product is intended
- (a) to meet the particular dietary requirements of an individual
- (i) who has a physical or physiological condition as a result of a disease, disorder or injury, or
- (ii) for whom a particular effect, including weight loss, is to be obtained by a controlled intake of food; or
- (b) for consumption by individuals who are under 18 years of age.
Representation — alcoholic beverages
132.3 It is prohibited to make an express or implied representation, including by way of a brand element, on a cannabis product — or on the package, label or panel of a cannabis product — if there are reasonable grounds to believe that the representation associates the cannabis product or the brand element with an alcoholic beverage.
Standardized cannabis symbol on vaping products
132.31 (1) The standardized cannabis symbol that must be obtained from the Minister in the form of an electronic file must be clearly and prominently displayed on the outer surface of a cannabis accessory that contains a cannabis extract and that is a cannabis product intended for inhalation if the cannabis extract contains THC in a concentration greater than 10 μg/g, taking into account the potential to convert THCA into THC.
Requirements
(2) The standardized cannabis symbol must meet the following conditions:
- (a) it must be at least 1.27 cm by 1.27 cm in size;
- (b) it must be displayed with a white border of at least 2 points on all sides; and
- (c) if a change is made to the size of the symbol, its dimensions must be proportional vertically and horizontally.
39 Section 133 and 134 of the Regulations are replaced by the following:
- Net weight and volume
133 The net weight and net volume that must be included on the label of a cannabis product in accordance with sections 124, 125, 132.1, 132.11, 132.14, 132.15, 132.17 and 132.18 must be within the tolerance limits set out for that product in the document entitled Tolerance Limits for the Net Weight and Volume Declared on Cannabis Product Labelling, as amended from time to time and published by the Government of Canada on its website.
Number of discrete units
134 The number of discrete units in a container that is labelled in accordance with sections 124, 132.1, 132.14 and 132.17 must be equal to the number specified on the label.
40 Section 139 of the Regulations is amended by adding the following in alphabetical order:
- common name has the same meaning as in subsection C.01.001(1) of the Food and Drug Regulations. (nom usuel)
41 Section 141 of the Regulations is repealed.
42 Paragraph 155(a) of the Regulations is replaced by the following:
- (a) the applicant does not hold an establishment licence that is necessary to authorize them to conduct, at the site or a building proposed in the application, the activities in relation to drugs containing cannabis that they intend to conduct there;
- (a.1) the Minister of Health suspends, in respect of one or more activities that the applicant intends to conduct in relation to drugs containing cannabis, an establishment licence that is necessary to authorize the applicant to conduct that activity at the site proposed in the application or at a building within the site;
43 Paragraph 156(a) of the Regulations is replaced by the following:
- (a) the Minister of Health suspends, in respect of one or more activities that the holder of the cannabis drug licence is authorized to conduct under the licence, an establishment licence that is necessary to authorize the holder to conduct that activity at the site or at a building within the site;
44 Paragraph 157(c) of the Regulations is replaced by the following:
- (c) the holder of the cannabis drug licence no longer holds an establishment licence that is necessary to authorize them to conduct, at the site or at a building within the site, one or more activities that are authorized under the cannabis drug licence; and
45 Section 196 of the Regulations is amended by adding the following in alphabetical order:
common name has the same meaning as in subsection C.01.001(1) of the Food and Drug Regulations. (nom usuel)
46 Subparagraph 205(c)(i) of the English version of the Regulations is replaced by the following:
- (i) a description of the cannabis,
47 Subparagraph 209(c)(i) of the English version of the Regulations is replaced by the following:
- (i) a description of the cannabis,
48 Subparagraph 214(c)(i) of the English version of the Regulations is replaced by the following:
- (i) a description of the cannabis,
49 Subparagraph 218(c)(i) of the English version of the Regulations is replaced by the following:
- (i) a description of the cannabis,
50 (1) The portion of subsection 224(1) of the Regulations before paragraph (a) is replaced by the following:
Inventory
224 (1) A holder of a licence must retain, for each lot or batch of cannabis — other than a cannabis extract, cannabis topical or edible cannabis — that they produce, a document that contains the following information, as applicable:
(2) Subsection 224(1) of the Regulations is amended by striking out "and" at the end of paragraph (e), by adding "and" at the end of paragraph (f) and by adding the following after paragraph (f):
- (g) except in the case of cannabis plants or cannabis plant seeds, any information that is obtained through testing and that relates to the phytocannabinoid and terpene content of the cannabis.
(3) The portion of subsection 224(2) of the Regulations before paragraph (a) is replaced by the following:
Packaging
(2) A holder of a licence must retain, for each lot or batch of cannabis — other than a cannabis extract, cannabis topical or edible cannabis — that they package, a document that contains the following information:
51 Subsections 225(1) and (2) of the Regulations are replaced by the following:
Inventory — cannabis extract, etc.
225 (1) A holder of a licence must retain, for each lot or batch of cannabis extract, cannabis topical or edible cannabis that they produce, a document that contains the following information:
- (a) the date of production and the net weight or volume of the cannabis extract, cannabis topical or edible cannabis on that date;
- (b) if applicable, the date on which the cannabis extract, cannabis topical or edible cannabis is put into a discrete unit form, the net weight or volume of each unit and the number of units;
- (c) in respect of the cannabis that is used to produce the cannabis extract, cannabis topical or edible cannabis,
- (i) its description,
- (ii) its net weight or volume,
- (iii) its lot or batch number, and
- (iv) the date on which it was produced;
- (d) in the case where the cannabis extract, cannabis topical or edible cannabis is, or will become, a cannabis product or is, or will be, contained in a cannabis accessory that is, or will become, a cannabis product,
- (i) the list of ingredients that is required to appear on the label of the cannabis product, and
- (ii) the net weight, net volume or concentration by weight or volume of each of these ingredients;
- (e) in the case of a cannabis extract that is, or will become, a cannabis product or that is, or will be, contained in a cannabis accessory that is, or will become, a cannabis product,
- (i) an indication of whether each ingredient is a carrier substance, flavouring agent or substance that is necessary to maintain the quality or stability of the cannabis product,
- (ii) any additional information in the possession of the holder that relates to the purpose of each ingredient, and
- (iii) a description of the flavour, if any, of the cannabis product; and
- (f) any information that is obtained through testing and that relates to the phytocannabinoid and terpene content of the cannabis extract, cannabis topical or edible cannabis.
Exception
(1.1) The document is not required to contain the information referred to in subparagraph (1)(d)(ii) in respect of an ingredient if
- (a) the ingredient is part of a mixture of substances that was used in the production of cannabis referred to in paragraph (1)(d);
- (b) the holder obtained the mixture from another person;
- (c) the information has not been disclosed to the holder;
- (d) the holder has made the necessary arrangements to ensure that the information will be provided to the Minister if, within the retention period referred to in subsection (3), the Minister requires the holder to provide it; and
- (e) the holder includes in the document the net weight or net volume of the mixture at the time at which it was used to produce the cannabis.
Packaging
(2) A holder of a licence must retain, for each lot or batch of cannabis extract, cannabis topical or edible cannabis that they package, a document that contains the following information:
- (a) a description of the cannabis extract, cannabis topical or edible cannabis, including the brand name, if applicable;
- (b) the date on which the cannabis extract, cannabis topical or edible cannabis is packaged and its net weight or volume on that date; and
- (c) in the case of a drug containing cannabis, the strength per unit of the drug.
52 Subsection 226(1) of the Regulations is replaced by the following:
Cannabis obtained from another person
226 (1) A holder of a licence must, if they obtain cannabis from another person, retain a document that contains the following information:
- (a) the name of the person from which the cannabis is obtained;
- (b) the address of the location at which the cannabis is obtained and, if that location is different from the site or sites at which the cannabis was produced, the address of the site or sites, if known;
- (c) the date on which the cannabis is obtained;
- (d) the quantity of cannabis that is obtained;
- (e) a description of the cannabis, including, if applicable, the brand name;
- (f) the lot or batch number of the cannabis;
- (g) in the case of a drug containing cannabis, the form of the drug and its strength per unit; and
- (h) in the case of cannabis plants, cannabis plant seeds or cannabis that is not of a class of cannabis set out in Schedule 4 to the Act, the intended use.
53 The Regulations are amended by adding the following after section 226:
Things to be used as ingredients
226.1 (1) A holder of a licence for processing must retain a document that contains the following information if the holder obtains or produces anything that will be used as an ingredient to produce a cannabis extract, a cannabis topical or edible cannabis:
- (a) the name and business address of the person, if any, who supplies the thing;
- (b) the date on which the holder takes possession of the thing or, if the thing is produced by the holder, the date on which production is completed;
- (c) a description of the thing, including the name by which it is generally known and, if applicable,
- (i) its chemical name,
- (ii) its common name, if that name is not the name by which it is generally known,
- (iii) its INCI name, and
- (iv) its CAS registry number; and
- (d) any lot code or other unique identifier that enables the thing to be traced.
Retention period
(2) The document must be retained for at least two years after the day on which it is prepared.
Definitions
(3) The following definitions apply in paragraph (1)(c).
- CAS registry number means the identification number assigned to a chemical by the Chemical Abstracts Service, a division of the American Chemical Society. (numéro d'enregistrement CAS)
- common name has the same meaning as in subsection B.01.001(1) of the Food and Drug Regulations. (nom usuel)
- INCI name has the same meaning as in subsection 2(1) of the Cosmetic Regulations. (appellation INCI)
54 Subsection 227(1) of the Regulations is amended by adding the following after paragraph (f):
- (f.1) in the case of a cannabis extract, cannabis topical or edible cannabis that is a cannabis product or that is contained in a cannabis accessory that is a cannabis product, the list of ingredients that appears on the label of the cannabis product;
55 Paragraph 229(1)(b) of the Regulations is replaced by the following:
- (b) the date on which the cannabis is destroyed and its pre-destruction net weight or net volume on that date;
56 (1) Paragraph 231(1)(a) of the Regulations is replaced by the following:
- (a) for each lot or batch of cannabis any portion of which has been sold or exported, retain a document demonstrating that the cannabis was produced, packaged, labelled, distributed, stored, sampled and tested in accordance with the provisions of Parts 5 and 6;
(2) Subparagraph 231(1)(e)(ii) of the Regulations is replaced by the following:
- (ii) a document that describes every investigation conducted under paragraph 19(2)(b) or (c) and any measures taken under that paragraph; and
57 Subsection 235(1) of the Regulations is replaced by the following:
System of control
235 (1) A holder of a licence other than a cannabis drug licence must retain, for each lot or batch of cannabis that they sell or distribute, a document that contains the information that is necessary for the system of control referred to in subsection 46(1).
Transitional Provisions
Exemption — cannabis oil
58 (1) A holder of a licence for processing or a licence for sale is — in respect of their activities in relation to cannabis oil, including in respect of ingredients and things to be used as ingredients — exempt from the application of the Cannabis Regulations if
- (a) the holder was, on the day before the day on which this section comes into force, authorized to conduct activities in relation to cannabis oil under such a licence; and
- (b) the holder conducts the activities in accordance with the Cannabis Regulations as they read immediately before the day on which this section comes into force.
Persons authorized to sell under provincial Act
(2) A person that is authorized under subsection 69(1) of the Act to sell cannabis is — in respect of the sale or distribution of cannabis oil that they obtain, directly or indirectly, from a holder referred to in subsection (1) that meets the conditions set out in that subsection — exempt from the application of the Cannabis Regulations if the person complies with those Regulations as they read immediately before the day on which this section comes into force.
Packaging and labelling
(3) A person that is authorized to sell cannabis oil is, in respect of such sale, exempt from the application of section 25 of the Act if the cannabis oil is packaged and labelled in accordance with the Cannabis Regulations as they read immediately before the day on which this section comes into force.
Meaning of terms
(4) In this section and section 59, terms have the same meaning as in the Cannabis Regulations.
Cessation of effect
(5) This section ceases to have effect on the day that, in the sixth month after the month in which this section comes into force, has the same calendar number as the day on which it comes into force or, if that sixth month has no day with that number, the last day of that sixth month.
Exemption — dried or fresh cannabis
59 (1) Dried or fresh cannabis is exempt from the application of subsection 93(3) of the Cannabis Regulations if the dried or fresh cannabis meets the requirements of subsection 94(1) of those Regulations, as it read immediately before the day on which this section comes into force.
Cessation of effect
(2) This section ceases to have effect on the day that, in the sixth month after the month in which this section comes into force, has the same calendar number as the day on which it comes into force or, if that sixth month has no day with that number, the last day of that sixth month.
Coming into Force
Coming into force
60 (1) Subject to subsection (2), these Regulations come into force on the day on which section 193.1 of the Cannabis Act comes into force, but if they are registered after that day, they come into force on the day on which they are registered.
Six months later
(2) Subsection 1(2) comes into force on the day that, in the sixth month after the month in which the other provisions of these Regulations come into force, has the same calendar number as the day on which those provisions come into force or, if that sixth month has no day with that number, the last day of that sixth month.