Vol. 151, No. 4 — February 22, 2017
Registration
SOR/2017-18 February 13, 2017
FOOD AND DRUGS ACT
CONTROLLED DRUGS AND SUBSTANCES ACT
Regulations Amending Certain Department of Health Regulations (Miscellaneous Program)
P.C. 2017-113 February 13, 2017
His Excellency the Governor General in Council, on the recommendation of the Minister of Health, makes the annexed Regulations Amending Certain Department of Health Regulations (Miscellaneous Program), pursuant to
- (a) subsection 30(1) (see footnote a) of the Food and Drugs Act (see footnote b); and
- (b) subsection 55(1) (see footnote c) of the Controlled Drugs and Substances Act (see footnote d).
Regulations Amending Certain Department of Health Regulations (Miscellaneous Program)
Food and Drugs Act
Food and Drug Regulations
1 Section A.01.042 of the English version of the Food and Drug Regulations (see footnote 1) is replaced by the following:
A.01.042 If an inspector examines or takes a sample of a food or drug under section A.01.041, the inspector may submit it to an analyst for analysis or examination.
2 Section A.01.043 of the Regulations is replaced by the following:
A.01.043 If an inspector, on examination of a sample of a food or drug or on receipt of a report of an analyst of the result of an analysis or examination of the sample, is of the opinion that the sale of the food or drug in Canada would constitute a violation of the Act or these Regulations, the inspector shall so notify in writing the collector of customs concerned and the importer.
3 Paragraphs B.14.007(a) and (b) of the French version of the Regulations are replaced by the following:
- a) lorsqu’il est vendu pour servir dans les viandes conservées et dans les sous-produits de viande conservés, il peut renfermer de l’acide ascorbique, de l’acide érythorbique, de l’acide isoascorbique, de l’ascorbate de calcium, de l’ascorbate de sodium, du carbonate de sodium, de l’érythorbate de sodium, de l’iso-ascorbate de sodium, du nitrate de potassium, du nitrite de potassium, du nitrate de sodium ou du nitrite de sodium, pourvu que ces nitrates et nitrites, le cas échéant, soient emballés séparément des épices et condiments;
- b) lorsqu’il est vendu pour servir dans la viande préparée ou dans les sous-produits de viande dans lesquels il est permis d’ajouter un agent gélatinisant, il peut renfermer un tel agent gélatinisant;
4 The portion of section B.14.009 of the French version of the Regulations before paragraph (a) is replaced by the following:
B.14.009 La marinade, la saumure et le mélange de salaison à sec employés dans le marinage des viandes conservées et des sous-produits de viande conservés peuvent renfermer
5 The portion of section B.14.031 of the French version of the Regulations before paragraph (a) is replaced by the following:
B.14.031 La viande conservée et les sous-produits de viande conservés sont faits de viande crue ou cuite ou d’un sous-produit de viande crue ou cuite, qui ont été salés, asséchés, marinés, saumurés ou fumés et peuvent être garnis d’une glace et renfermer
6 Paragraph B.21.003(b) of the French version of the Regulations is replaced by the following:
- b) s’il est congelé, être enrobé d’une glaçure se composant d’eau, de monoglycérides acétyles, de chlorure de calcium, d’alginate de sodium, de carboxyméthycellulose de sodium, de phosphate disodique, de sirop de maïs, de dextrose, de glucose, de solides du glucose, d’acide ascorbique ou de son sel de sodium, ou d’acide érythorbique ou de son sel de sodium;
7 The portion of section B.21.005 of the French version of the Regulations before paragraph (a) is replaced by the following:
B.21.005 Le poisson, à l’exception des protéines de poisson, les produits de chair ou leurs préparations sont falsifiées si l’une des substances ci-après ou une substance de l’une des catégories ci-après s’y trouve ou y a été ajoutée :
8 The portion of section B.21.006 of the Regulations before paragraph (a) is replaced by the following:
B.21.006 Prepared fish or prepared meat shall be the whole or minced food prepared from fresh or preserved fish or meat respectively, may be canned or cooked, and may,
9 Paragraph B.21.006(n) of the French version of the Regulations is replaced by the following:
- n) dans le cas d’un mélange de poisson et de chair préparés qui a l’apparence et le goût de la chair d’animaux marins ou d’animaux d’eau douce, contenir du remplissage, un liant à poisson, de l’œuf entier, du blanc d’œuf, du jaune d’œuf, un colorant alimentaire, des agents gélatinisants ou stabilisants, des agents modifiant la texture, des préparations aromatisantes naturelles, des préparations aromatisantes artificielles, des agents rajusteurs du pH, de l’édulcorant et, dans une proportion ne dépassant pas deux pour cent du mélange, des légumineuses;
10 Section B.21.007 of the French version of the Regulations is replaced by the following:
B.21.007 Le liant à poisson devant servir dans ou sur le poisson ou la chair préparés doit être du remplissage auquel on a ajouté n’importe quel mélange de sel, de sucre, de dextrose, de glucose, d’épices ou d’autres condiments.
11 The portion of section B.21.021 of the French version of the Regulations before paragraph (a) is replaced by the following:
B.21.021 Le poisson conservé et la chair conservée doivent être du poisson ou de la chair, à l’état cru ou cuit, qui ont été desséchés, salés, marinés, saumurés ou fumés; ils peuvent renfermer un agent de conservation de la catégorie I, un agent de conservation de la catégorie II, du dextrose, du glucose, des épices, du sucre et du vinaigre, et :
12 The portion of section B.22.021 of the French version of the Regulations before paragraph (a) is replaced by the following:
B.22.021 La viande de volaille conservée et les sous-produits de viande de volaille conservés sont de la viande de volaille ou des sous-produits de viande de volaille crus ou cuits, qui ont été salés ou fumés et qui peuvent renfermer :
13 Section C.01.009 of the Regulations is replaced by the following:
C.01.009 If any Act of Parliament or any of its regulations prescribes a standard or grade for a drug and that standard or grade is given a name or designation in the Act or regulation, no person shall, on a label of or in any advertisement for that drug, use that name or designation unless the drug conforms with the standard or grade.
14 (1) Subsection C.01.019(2) of the French version of the Regulations is replaced by the following:
(2) Le rapport comprend une analyse critique et concise des réactions indésirables à la drogue et des réactions indésirables graves à la drogue, ainsi que les fiches d’observation portant sur toutes les réactions indésirables à la drogue et les réactions indésirables graves à la drogue — ou celles qui sont précisées par le ministre — qui sont connues du fabricant et qui sont associées au sujet de préoccupation que le ministre a demandé à celui-ci d’analyser dans le rapport.
(2) Subsection C.01.019(3) of the Regulations is replaced by the following:
(3) The Minister shall, after giving the manufacturer an opportunity to be heard, specify a period for the submission of the report that is reasonable in the circumstances. The Minister may only specify a period that is less than 30 days if the Minister needs the information in the report to determine whether the drug poses a serious and imminent risk to human health.
15 Paragraph C.01A.013(a) of the Regulations is replaced by the following:
- (a) there is any change to the information referred to in any of paragraphs C.01A.005(a), (b), and (e) to (i); or
16 Subsection C.05.014(3) of the Regulations is replaced by the following:
(3) Sections C.01.016 to C.01.020 do not apply to drugs used for the purposes of a clinical trial.
17 The Regulations are amended by replacing “comminuted” with “minced” in the following provisions:
- (a) paragraph B.21.006(s); and
- (b) paragraph B.21.021(e).
18 The French version of the Regulations is amended by replacing “produit du mélange et du malaxage” with “produit du râpage et du mélange” in the following provisions:
- (a) subparagraphs B.08.040(1)(a)(i) and (ii);
- (b) subparagraph B.08.041(1)(a)(i);
- (c) subparagraph B.08.041.1(1)(a)(i);
- (d) subparagraph B.08.041.2(1)(a)(i);
- (e) subparagraph B.08.041.3(1)(a)(i);
- (f) subparagraph B.08.041.4(1)(a)(i);
- (g) subparagraphs B.08.041.5(1)(a)(i) and (ii);
- (h) subparagraph B.08.041.6(1)(a)(i);
- (i) subparagraph B.08.041.7(1)(a)(i); and
- (j) subparagraph B.08.041.8(1)(a)(i).
19 The French version of the Regulations is amended by replacing “hachée finement” with “hachée” in the following provisions:
- (a) section B.14.033;
- (b) section B.14.035; and
- (c) paragraph B.14.037(1)(a).
20 The French version of the Regulations is amended by replacing “chair de poisson” with “chair” in the following provisions:
- (a) subparagraph B.21.005(b)(ii);
- (b) the portion of section B.21.020 before paragraph (a); and
- (c) paragraph B.21.021(c).
21 The French version of the Regulations is amended by replacing “trouble” with “désordre” in the following provisions:
- (a) section C.01.010; and
- (b) subparagraphs C.01.040.3(a)(i) and (ii).
Medical Devices Regulations
22 Section 5 of the Medical Devices Regulations (see footnote 2) is replaced by the following:
5 These Regulations do not apply to a medical gas piping system that is assembled on site at a health care facility and permanently built into the structure of the facility.
Regulations Amending the Food and Drug Regulations (Labelling, Packaging and Brand Names of Drugs for Human Use)
23 Section 5 of the Regulations Amending the Food and Drug Regulations (Labelling, Packaging and Brand Names of Drugs for Human Use) (see footnote 3) is amended by replacing the portion of subsection C.01.004.02(4) before paragraph (a) that it enacts with the following:
(4) If the composition of the drug varies from one lot to another with respect to its non-medicinal ingredients,
Controlled Drugs and Substances Act
Narcotic Control Regulations
24 Section 28 of the Narcotic Control Regulations (see footnote 4) is replaced by the following:
28 A licensed dealer must not sell or provide a narcotic, other than a preparation described in section 36, unless the narcotic is securely packed in its immediate container. The container must be sealed in such a manner that it cannot be opened without breaking the seal.
25 Paragraphs 59(4)(a.1) and (a.2) of the Regulations are replaced by the following:
- (a.1) has performed an activity referred to in section 7 of the Access to Cannabis for Medical Purposes Regulations in regard to a person who is not under their professional treatment;
- (a.2) has contravened section 8 or 9 of those Regulations;
Access to Cannabis for Medical Purposes Regulations
26 Section 88 of the Access to Cannabis for Medical Purposes Regulations (see footnote 5) is replaced by the following:
88 In the case of fresh or dried marihuana, cannabis oil or marihuana plants or seeds to be sold or provided to a client or an individual who is responsible for the client, the information required under section 84, 85 or 86 and under paragraph 87(1)(a) or subsection 87(2) or (3), as applicable, may be set out on one label.
27 The portion of subsection 90(1) of the Regulations before paragraph (a) is replaced by the following:
90(1) All information that is required under section 84, 85 or 86 and under paragraph 87(1)(a) or subsection 87(2) or (3), as applicable, to appear on a label must be
Coming into Force
28 These Regulations come into force on the day on which they are registered.
REGULATORY IMPACT ANALYSIS STATEMENT
(This statement is not part of the Regulations.)
Issues
Health Canada (the Department) and the Standing Joint Committee for the Scrutiny of Regulations (SJCSR) identified miscellaneous minor amendments required for the Food and Drug Regulations (FDR), Medical Devices Regulations (MDR) and Narcotics Control Regulations (NCR). Further, the SJCSR indicated that the repeal of a redundant subsection to the NCR should be made. In addition, Health Canada identified the need to correct minor provision numbering errors in the Access to Cannabis for Medical Purposes Regulations (ACMPR).
These amendments, which are being brought forward by the Department of Health, have been collected in this omnibus Regulatory Impact Analysis Statement (RIAS).
Objective
These amendments have the following objectives:
- to correct references to section numbering;
- to correct a discrepancy between the French and English versions;
- to harmonize terms used in the regulations with those used in the enabling statute;
- to add clarity to a regulatory provision;
- to correct typographical or grammatical errors; and
- to repeal obsolete, spent or redundant regulatory provisions.
Description
Amendments to the FDR:
1. Correct typographical or grammatical errors:
- B.14.033, B.14.035 and B.14.037(1)(a): “hachée finement” will be changed to the correct tense of “hachée.”
- In Part B, the terms “preserve,” “preserved” and “preserves” appear in a total of 145 instances when referring to meat, poultry, and marine and fresh water animal products (e.g. fish). The Department has analyzed whether the French translation of each of these instances is using the correct tense of the verb “conserver.” In eight provisions the translations have been found to be incorrect and they will be changed to the proper tense.
- B.21.002(d): The French grammar inaccuracy “animaux marine” will be changed to “animaux marins.”
2. Add clarity to regulatory provisions:
- C.01.019(3): This provision will be amended to add the word “only” in the English version and “ne . . . que” in the French version to clarify intent.
- C.01.004.02(4): This provision will be amended to clarify the intent of the provision by adding the text in bold in both the English and French versions: “If the composition of a drug varies from one lot to another with respect to its non-medicinal ingredients.”
3. Harmonize terms used in the Regulations with those used in the enabling statute:
- C.01.010 and C.01.040.03(a): In the French version, the word “trouble” will be changed to “désordre” to align with terminology in the Food and Drugs Act.
4. Correct discrepancies between French and English versions noted by the SJCSR:
- B.08.040 to B.08.041.8: The French translation of “the product made by comminuting and mixing” (currently “produit du mélange et du malaxage”) will be changed to “produit du râpage et du mélange.”
- B.21.006[S], B.21.006(s) and B.21.021(e): When the terms are used to describe fish or seafood, all instances of “comminuted” will be changed to “minced” and all instances of “déchiquetés” will be changed to “hachés.” When “comminuted” and “déchiquetés” are used in reference to foods that are not fish or seafood, they will remain unchanged. This is consistent with the repeal of the term “comminuted” from the Canadian Food Inspection Agency’s Fish Inspection Regulations and will result in the same term used in the French translation (haché) for both “comminuted” and “minced.”
- As per the Department’s interpretation that “meat” is “chair” in French as it pertains to marine and fresh water animal products:
- B.21.005(b)(ii), B.21.020, B.21.021, B.21.021(c): “chair de poisson” will be changed to “chair.”
- B.21.006[S], B.21.006(n), B.21.007[S]: “viande” will be changed to “chair.”
- C.01.019(2): The French text “tout ou partie des réactions indésirables” will be changed to better align with the English “all or specified adverse drug reactions.”
5. Correct discrepancy between French and English versions identified by Health Canada:
- A.01.043: will be changed as follows:
- (EN)
 If an inspector, upon examination of a sample of a food or drug
If an inspector, upon examination of a sample of a food or drug  or on receipt of a report of an analyst of the result of an analysis or examination of the
or on receipt of a report of an analyst of the result of an analysis or examination of the  sample, is of the opinion that the sale of the food or drug in Canada would constitute a violation of the Act or these Regulations, the inspector shall ….
sample, is of the opinion that the sale of the food or drug in Canada would constitute a violation of the Act or these Regulations, the inspector shall …. - (FR) L’inspecteur qui estime, après examen d’un échantillon de l’aliment ou de la drogue ou réception du rapport de l’analyste que la vente de l’aliment ou de la drogue
 serait contraire à la Loi ou au présent règlement,
serait contraire à la Loi ou au présent règlement, 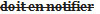 en avise ….
en avise ….
- (EN)
- B.21.003(b): The incorrect French translation of the word “glaze” (currently “lustre”) will be changed for “glaçure.”
- B.21.005: The words “d’animaux marins ou d’animaux d’eau douce ou leurs préparations” will be removed so that this section would simply refer to “chair.”
6. Correct section number referencing errors:
- There is an incorrect reference to adverse drug reporting requirements in the FDR that apply to drugs in clinical trials due to renumbering in Division 1 that came when C.01.018 to C.01.020 were added. The reference in subsection C.05.014(3) to “C.01.016 and C.01.017” will be changed to “C.01.016 to C.01.020” in the English version and from “C.01.016 et C.01.017” to “C.01.016 à C.01.020” in the French version.
- The reference in C.01A.013(a) to “subparagraphs C.01A.005(g)(i) and (ii)” is an error, given that paragraph (g) has been amended such that it no longer has subparagraphs. Paragraph C.01A.013(a) will be amended such that the reference is to C.01A.005(g).
7. Repeal obsolete provisions or modernize obsolete references or archaic language:
- The archaic language in C.01.009 of the FDR will be modernized by replacing “statute of the Parliament of Canada” by “Act of Parliament” in the English version, and by replacing “un statut du Parlement du Canada” by “une loi fédérale” and “d’un statut” by “d’une telle loi” in the French version.
Amendments to the MDR will:
1. Repeal obsolete provisions or modernize obsolete references or archaic language:
- Paragraphs 5(a) and 5(b) of the MDR will be repealed as they are obsolete standards. Medical gas pipelines will henceforth be regulated through national and provincial building codes, as they form part of the structure of a hospital. The Department’s regulatory authorities exempt medical gas pipelines that are built as part of the hospital structure from the MDR.
Amendments to the NCR will:
1. Repeal subsection 28(1) as its labelling requirements have been rendered redundant due to amendments in the FDR in 2013.
- The Controlled Drugs and Substances Act (CDSA) and its regulations, including the NCR, provide a framework for the control of substances that can alter mental processes and that may cause harm to health or to society when diverted to an illicit market or used inappropriately. Within this context, subsection 28(1) of the NCR states that:
- “No licensed dealer shall sell or provide a narcotic that is not a drug within the meaning of section 2 of the Food and Drugs Act, unless the narcotic is labelled in accordance with the Food and Drug Regulations.”
- Subsection 28(1) was intended to ensure that narcotics in the form of active pharmaceutical ingredients (rather than only finished drug products) were labelled according to the FDR. Due to amendments made to the FDR in 2013, however, subsection 28(1) of the NCR is unnecessary as the raw materials (i.e. active pharmaceutical ingredients) are now subject to labelling requirements set out in the FDR.
- In accordance with section 59 of the NCR, the Minister may notify certain authorities (e.g. licensed producers, health care licensing authorities) if the Minister has reasonable grounds to believe that a health care practitioner has contravened certain provisions in the ACMPR. The provisions referenced in section 59 as belonging to the ACMPR (i.e. sections 128, 129 and 130) refer to provisions as they were numbered in the former Marihuana for Medical Purposes Regulations (MMPR). These former MMPR provisions are now found in sections 7, 8 and 9 of the ACMPR. The proposed amendment will correct the references in this section of the Regulations.
Amendments to the ACMPR will correct numbering errors in:
1. Section 88
- This section is intended to allow licensed producers to combine on one label product information and client information for all products. Specific product and client labelling requirements vary depending on the product being sold or provided: fresh or dried marihuana or cannabis oil, or marihuana seeds and plants.
- Product labelling requirements for marihuana seeds and plants are set out in sections 85 and 86 respectively. Section 87 sets out the labelling requirements for clients.
- As the Regulations are currently written, licensed producers may not combine on one label product information related to marihuana seeds and plants with information about the client (as it is the case for fresh or dried marihuana or cannabis oil) because section 88 does not reference sections 85 and 86; however, this is not the intent. The proposed amendments will enable licensed producers to combine, on one label, product information related to marihuana seeds and plants with client information. This will correct this inconsistency in the Regulations and will be similar to the case for fresh or dried marihuana and cannabis oil.
2. Section 90
- This section of the ACMPR requires that all information on the product label for fresh and dried marihuana and cannabis oil and the client label for fresh and dried marihuana, cannabis oil, marihuana seeds and marihuana plants be in English and French, be clearly and prominently displayed on the label and be readily discernible under the customary conditions of use. The proposed amendments will enable these labelling requirements to also apply to information on the product label for marihuana seeds and plants, in addition to fresh and dried marihuana and cannabis oil, and correct this inconsistency among products.
“One-for-One” Rule
The “One-for-One” Rule does not apply to these amendments, as there is no change in administrative costs or burden to business.
Small business lens
The small business lens does not apply to these amendments, as there are no costs to small business.
Rationale
Some of the amendments to the FDR are in response to the SJCSR’s review of the Regulations. The need for a number of other minor technical amendments to the FDR (correction of language discrepancies, and referencing and typographical errors, modernizing obsolete references or archaic language) and to the MDR (repeal of obsolete provisions) have also been identified by the Department. The amendments help to correct or improve the regulatory base and do not impose any costs on the government or stakeholders.
The repeal of subsection 28(1) of the NCR responds to a concern raised by the SJCSR. This provision is no longer required after the coming into force of the Regulations Amending the Food and Drug Regulations (1475 — Good Manufacturing Practices) published in May 2013. With the 2013 amendments, certain provisions under the FDR pertaining to labelling requirements were extended to be applicable for both active ingredients and finished products. The labelling requirements of subsection 28(1) of the NCR are no longer required.
The amendments to the ACMRP and other amendments to the NCR correct regulatory drafting errors, ensure consistency and clarity in the regulatory framework for cannabis for medical purposes, and do not impose any costs on the government or stakeholders.
Contacts
Nancy Maguire
Manager
Departmental Regulatory Affairs
Regulatory Operations and Regions Branch
Health Canada
Address Locator: 3005A
Holland Cross, Tower B, 5th Floor
11 Holland Avenue
Ottawa, Ontario
K1A 0K9
Email: DRA-ARM@hc-sc.gc.ca
Amendments to the FDR and MDR:
Bruno Rodrigue
Director
Office of Legislative and Regulatory Modernization
Policy, Planning and International Affairs Directorate
Health Products and Food Branch
Health Canada
Address Locator: 3000A
Holland Cross, Tower A, Suite 14
11 Holland Avenue
Ottawa, Ontario
K1A 0K9
Email: LRM_MLR_consultations@hc-sc.gc.ca
Amendments to the ACMPR:
Office of Medical Cannabis
Health Canada
Address Locator: 0302B
Ottawa, Ontario
K1A 0K9
Email: OMC-Engagement-BCM@hc-sc.gc.ca
Amendments to the NCR:
Denis Arsenault
Manager
Healthy Environments and Consumer Safety Branch
Health Canada
Main Stats Building
150 Tunney’s Pasture Driveway
Ottawa, Ontario
K1A 0K9
Email: OCS_regulatorypolicy-BSC_ politiquereglementaire@hc-sc.gc.ca
- Footnote a
S.C. 2012, c. 19, s. 414 - Footnote b
R.S., c. F-27 - Footnote c
S.C. 2015, c. 22, s. 4(1) - Footnote d
S.C. 1996, c. 19 - Footnote 1
C.R.C., c. 870 - Footnote 2
SOR/98-282 - Footnote 3
SOR/2014-158 - Footnote 4
C.R.C., c. 1041 - Footnote 5
SOR/2016-230